Cryptotis nigrescens ( Allen, 1895 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/sead011 |
|
persistent identifier |
https://treatment.plazi.org/id/03F787A7-FFE1-FFA1-FF6B-B61DA198FE84 |
|
treatment provided by |
Felipe |
|
scientific name |
Cryptotis nigrescens ( Allen, 1895 ) |
| status |
|
Cryptotis nigrescens ( Allen, 1895) View in CoL
Blackish Small-eared Shrew; Musaraña Negruzca de Orejas Pequeñas
Blarina micrura : Allen, 1893:238. Not Sorex micrurus Tomes, 1861:279 = Blarina tropicalis Merriam, 1895:21 (= Cryptotis tropicalis View in CoL ); name preoccupied by G [alemys]. micrurus Pomel, 1848:249 = Sorex talpoides Gapper,1830:202 = Blarina brevicauda talpoides View in CoL .
Blarina (Soriciscus) nigrescens Allen,1895:339 . Type locality “San Isidro (San José), Costa Rica.” Holotype: skin and skull of an individual of unknown sex, AMNH (American Museum of Natural History) number 9591/7952.
C [ryptotis]. nigrescens : Miller, 1911:222. First use of current name combination.
Cryptotis zeteki Setzer, 1950:299 . Type locality “Cerro Punta (lat. 8º42 ʹ N, long. 82º48 ʹ W), 6,500 feet, Chiriquí Province, Republic of Panama.” Holotype: skin and skull of an adult female, USNM ( U.S. National Museum of Natural History) number 290466.
Cryptotis tersus Goodwin, 1954:1 . Type locality “Santa Clara, 4200 feet elevation, on the Pan American Highway, 15 miles from the border of Costa Rica, Chiriquí Province, Republic of Panama.” Holotype: skin and skull of an adult male, AMNH number 164695.
C [ryptotis]. nigricans: Goodwin, 1954:2. Incorrect subsequent spelling of Blarina nigrescens Allen, 1895 (= Cryptotis nigrescens View in CoL ).
Cryptotis nigrescens zeteki : Handley, 1966:756. Name combination.
Cryptotis parva orophila View in CoL : Choate, 1970:262. Part, not Blarina (Soriciscus) orophila Allen, 1895 (= Cryptotis orophilus ).
Cryptotis nigrescens nigrescens View in CoL : Choate, 1970:279. Name combination.
CONTEXT AND CONTENT. Cryptotis nigrescens View in CoL is monotypic; subspecies previously recognized as comprising C. nigrescens View in CoL (e.g., C. n. mayensis View in CoL , C. n. merriami View in CoL —see Choate, 1970) are now recognized as separate species ( Woodman and Timm 1993; Burgin 2018; Woodman 2018). Cryptotis nigrescens View in CoL is a member of the C. nigrescens View in CoL group, an informal morphological grouping ( Choate 1970; Woodman 2018) and genetic clade ( He et al. 2015; Baird et al. 2017; Mejía-Fontecha et al. 2021) that includes the Eastern Cordillera small-eared shrew, C. brachyonyx Woodman, 2003 View in CoL ; Colombian small-eared shrew, C. colombianus Woodman and Timm, 1993 ; Honduran small-eared shrew, C. hondurensis Woodman and Timm, 1992 View in CoL ; Lacandona small-eared shrew, C. lacandonensis Guevara et al., 2014 ; Yucatan small-eared shrew, C. mayensis ( Merriam, 1901) View in CoL ; Merriam’s small-eared shrew, C. merriami Choate, 1970 View in CoL ; and Darién small-eared shrew, C. merus Goldman, 1912 . The synonymies are adapted and updated from Woodman and Timm (1993) and Woodman (2018).
DIAGNOSIS
Cryptotis nigrescens is a small- to medium-sized member of the genus as measured by head–body length, condylobasal length of the skull (CBL), and mass ( Table 1). It has dark blackishbrown dorsal pelage, which exhibits little contrast with its ventral pelage, and a moderately long tail that typically averages about 42% of head–body length (Fig. 1; Table 1). Five other species of small-eared shrews are known to occur in Costa Rica and western Panama ( Table 2). Cryptotis nigrescens is much smaller in head– body length and condylobasal length, has a much shorter tail, and a shorter, less robust humerus than either Ender’s small-eared shrew, C. endersi Setzer, 1950 , or the Monteverde small-eared shrew, C. monteverdensis Woodman and Timm, 2017 . From the Talamancan small-eared shrew, C. gracilis Miller, 1911 , C. nigrescens can be distinguished by its much shorter relative tail length, broader zygomatic plate, shorter and broader rostrum, higher coronoid process of the dentary, and less robust humerus with smaller processes; from the Central American least shrew, C. orophilus ( Allen, 1895) , by its much darker dorsal and ventral pelage, much longer relative tail length, and relatively broader zygomatic plate; and from C. merriami by its darker pelage, lack of bulbous dentition, and relatively shorter and narrower palate ( Woodman and Timm 1993, 2017; Woodman 2000; Pine et al. 2002; Woodman and Gaffney 2014).
GENERAL CHARACTERS
Terminology for dentition and dental characteristics follows Choate (1970); for cranial anatomy and measurements, Woodman and Timm (1993, 1999); for postcranial anatomy, Reed (1951). A mensural character is considered “very small” if its mean is ≥1 SD below the mean for the genus Cryptotis , “small” if its mean is 0.5–1.0 SD below the mean for the genus, “moderate” if within ± 0.5 SD of the genus mean, “large” if 0.5– 1.0 SD above the genus mean, and “very large” if ≥1 SD above the genus mean. The mean for the genus was calculated from mean measurements from up to 62 species, subspecies, and distinctive populations of Cryptotis . Most measurements reported herein are from the authors.
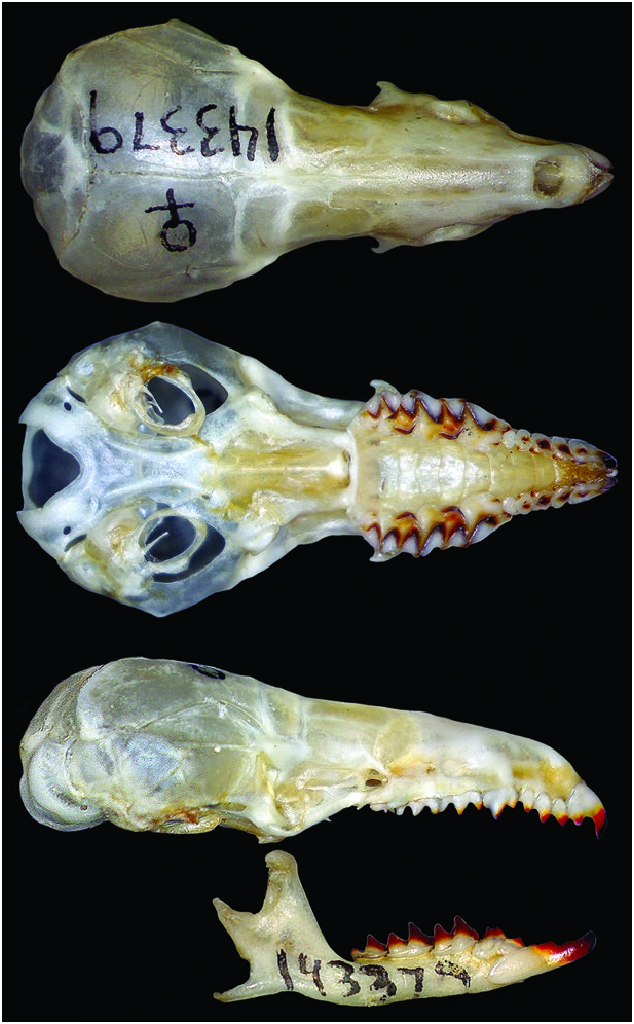
Like other Blarinini shrews, Cryptotis nigrescens is small with a fusiform body shape, elongate and pointed rostrum, minute eyes, and pinnae reduced to slit-like openings that are hidden by its fur (Fig. 1). Among the small-eared shrews of the genus Cryptotis , it is small to medium in body size, with head–body length averaging 68 mm and tail averaging 28 mm in length or 42% of head–body length ( Table 1). Dorsal hairs are approximately 4.5 mm long (range: 4–5 mm) and appear two-banded. Pelage is dark; dorsal and lateral pelage Mummy Brown or Clove Brown; ventral pelage slightly paler— Buffy Brown, Saccardo’s Umber, Olive Brown, or Mouse Gray (colors after Ridgway 1912). Video by James Wolfe taken 2.3 km NNW of St. Elena, Costa Rica, and illustrating the external characteristics and foraging behavior of C. nigrescens can be found at https:// mammalsofcostarica.com/order-eulipotyphia-shrews/. Two videos of a live C. nigrescens taken at 0830 h on 24 April 2020 by Wilson Salas along the Nuboso Trail in the Monteverde Cloud Forest Reserve, Costa Rica, are referenced in Supplementary Data SD1 and SD2.
The species has a short skull, condylobasal length (CBL) averaging 18.4 ± 0.7 mm ( Fig. 2 View Fig ); relatively short rostrum (palatal length [PL] averages 42.7 ± 0.7% of CBL, n = 107); moderately broad interorbital breadth (23.6 ± 0.9% of CBL, n = 107) and braincase (49.9 ± 1.2% of CBL, n = 100); often two dorsal foramina (68%, n = 59), small to large in size and positioned close to suture between frontals; typically no foramen posterior to the dorsal articular facet (88%, n = 57); generally a well-developed foramen dorsal to external capitular facet on one (2%, n = 57) or both sides of the skull (93%); however, this foramen may be minute (3%); lacks a large, obvious foramen on the posterior edge of the tympanic process of the petromastoids; zygomatic plate broad in proportion to CBL (10.6 ± 0.8%, n = 108), very broad in proportion to PL (24.8 ± 2.1%, n = 119); anterior border of zygomatic plate aligned with mesostyle to mesostyle– metastyle valley of M1, posterior border with the posterior one-half of mesostyle–metastyle valley of M2 to middle of M3, and usually near the middle of the base of the maxillary process, but can be positioned from the anterior one-half of this process to posterior to the process; palate relatively broad (breadth across upper second molars averages 69.2 ± 2.1% of PL, n = 120).
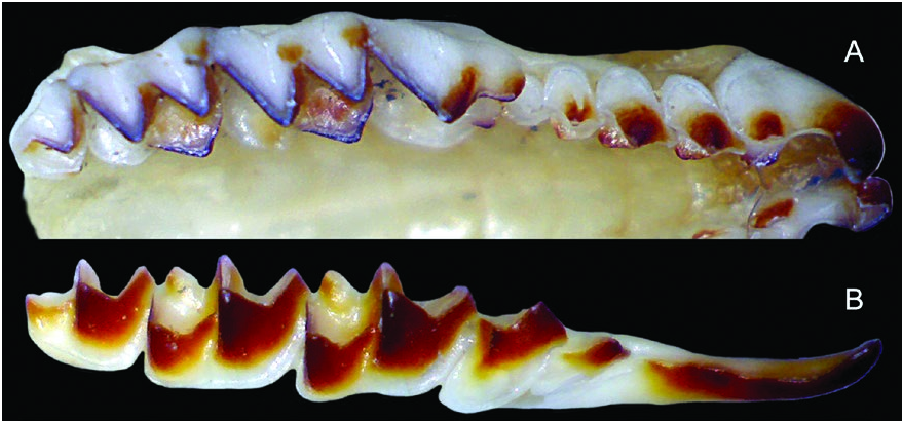
Dentition not bulbous; upper toothrow generally uncrowded ( Fig. 3 View Fig ); unicuspid toothrow moderately long (averaging 12.9 ± 0.6% of CBL, n = 108); upper fourth unicuspid (U4) typically in line with the rest of the unicuspid toothrow and preventing contact between the upper third unicuspid (U3) and the upper fourth premolar (P4); U4 usually obscured by P 4 in lateral view of the skull, but sometimes partly visible; posterior borders of P4, M1, and M2 unrecessed or only slightly recessed; M3 typically possesses a well-developed paracrista, and paracone, reduced precentrocrista and mesostyle, poorly developed, but often pigmented postcentrocrista and metacone (but postcentrocrista short with mesostyle and metacone closely associated), protocone present and often pigmented, and poorly developed hypocone often present.
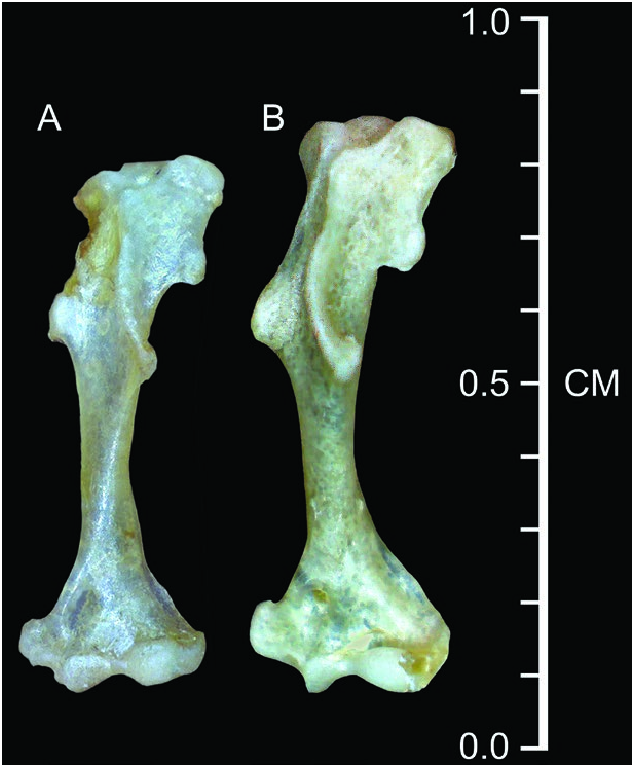
Dentary short (length = 6.2 ± 0.3%, n = 119); coronoid process low relative to dentary length (71.2 ± 2.6%, n = 117) and joins the horizontal ramus of the dentary at a steep angle; very short posterior dentary (72.6 ± 2.8% of dentary length, n = 119); articular condyle relatively short and narrow; lower sigmoid notch very shallow; posterior border of lower incisor reaches almost to posterior border of cingulum of p4; vestigial entoconid occasionally present in talonid of m3 (38%, n = 50). The humerus ( Fig. 4 View Fig ) appears relatively long and narrow with weakly developed processes ( Woodman and Timm 1993, 2017; Woodman 2000; Woodman and Gaffney 2014).
DISTRIBUTION
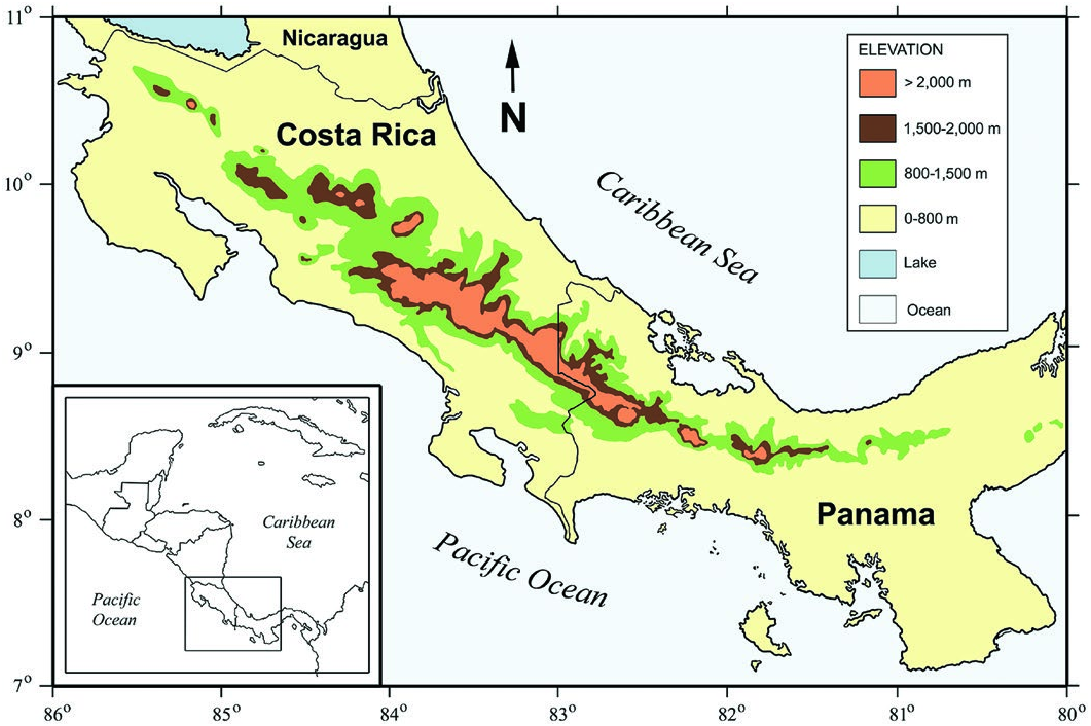
Cryptotis nigrescens occurs in highlands above 800 m in the
Tilarán, Central, and Talamanca cordilleras of Costa Rica and
55(1035)— Cryptotis nigrescens MAMMALIAN SPECIES 5
the Chiriquí Cordillera of western Panama ( Fig. 5 View Fig ). The recorded elevational distribution is 870–2,865 m in Costa Rica and 820– 2,400 m in Panama ( Woodman and Timm 1993; McCain 2004;
Timm and LaVal 2016). There is as yet no fossil record for C. nigrescens .
FORM AND FUNCTION
Like most other species of small-eared shrews that have been examined for sexual dimorphism, female and male Cryptotis nigrescens do not differ substantially in body size ( Table 3) and are difficult to distinguish externally. Woodman and Timm (1993) discovered no significant sexual dimorphism in external measurements within populations of C. nigrescens from Monteverde, Costa Rica (n = 16 females, 16 males), or from San Félix, Panama (n = 8 females, 9 males). Similarly, there were no significant differences between the sexes in multivariate size or shape of the cranium within either the Monteverde (n = 12 females, 14 males) or San Félix (n = 10 females, 13 males) populations ( Table 3).
Woodman and Timm (1993) demonstrated that the population of C. nigrescens from Monteverde in northwestern Costa Rica averaged distinctly smaller than populations from near San Félix, western Panama, and speculated that C. nigrescens might be a complex of species. Woodman (2000) subsequently documented possible clinal variation in size throughout the distribution of C. nigrescens , with western Panamanian populations averaging about 7% larger in head-and-body length and 5% larger in condylobasal length than populations in northern Costa Rica ( Table 1). See also the comparison of humeri in Fig. 4 View Fig .
Subtle geographic variation in pelage coloration has been reported, with shrews in the Monteverde population typically possessing slightly darker pelage than those in the San Félix population in the Chiriquí highlands of western Panama. The darkest pelage, however, is found among individuals from Fish Camp, Bocas del Toro Province, Panama ( Woodman and Timm 1993).
Lateral flank glands are often present in male and female soricids, and they are believed to play a role in reproductive behavior ( Hamilton 1929; Dryden and Conaway 1967; Hawes 1976). The glands are typically small and hidden by the pelage in females, whereas in mature males, the glands may be visible externally as oval patches of thickened skin covered by very short, pale bristles ( Johnsen 1914; Eadie 1938; Carraway 2009). Inspection of 26 male C. nigrescens from throughout the species’ geographic range showed well-developed lateral glands on 20 individuals collected in May through the beginning of July. Six males lacking well-developed lateral glands were obtained in February (n = 1), March (n = 2), April (n = 1), and July (n = 2— Woodman and Timm 1993).
The forelimb of C. nigrescens exhibits few of the structural modifications associated with semi-fossoriality that are seen in some other members of the genus, such as in the C. mexicanus group and the C. goldmani group (see Woodman and Gaffney 2014). The foreclaws are relatively short and narrow; the bones of the manus are long and narrow with the exception of the distal phalanges, which are short and narrow and support only about half the length of the foreclaws; the humerus is relatively long and narrow with relatively small processes for muscle attachments ( Fig. 4 View Fig ). Based on its limb morphology, locomotor behavior of C. nigrescens is classified as one of the most ambulatory species in the genus ( Woodman and Morgan 2005; Woodman and Gaffney 2014; Woodman 2023a). Individuals we observed running on the ground at Monteverde moved very quickly.
The axial skeleton of C. nigrescens exhibits some variation in number and position of vertebrae. Typically, there are seven cervical vertebrae (n = 16 Panama, 36 Costa Rica), although one Costa Rican specimen (KU [University of Kansas Biodiversity Institute] 143377) has a rib attached to the left side only of its seventh cervical vertebra. The modal number of thoracic vertebrae is 13, with ranges of 13–14 in Costa Rica (n = 36) and 12–14 in Panama (n = 14). The modal number of lumbar vertebrae is 6, with ranges of 5–6 in Costa Rica (n = 36) and 5–7 in Panama (n = 11). The mode of sacral vertebrae fused to each other at the centrum and sacral crest is four, with a range of 3–4 ( Costa Rica, n = 29; Panama, n = 15). Occasionally, the last lumbar vertebra is fused to one (USNM [National Museum of Natural History] 541028) or both (USNM 541034) innominates, but not to the sacrum. Caudal vertebrae exhibit notable variation in numbers between populations. In Costa Rica, the mode is 14 with a range of 14–16 (n = 19), whereas in Panama, the mode is 16 with a range of 14–16 (n = 8). Total numbers of vertebrae vary similarly. The mode in Costa Rica is 44 with a range of 44–46 (n = 16), and the mode in Panama is 46 with a range of 44–46 (n = 8). Numbers of ribs also vary, with a mode in both countries of 13 pairs. The range in Costa Rica is 13–14 pairs (n = 36; this total does not include one individual with 13 right and 14 left ribs: KU 143377), and the range in Panama is 14–15 pairs (n = 14).
A young adult female (KU 143393) captured at Monteverde on 15 May 1989 lacked digits on its right forefoot. The vestigial first digit was the only one bearing a claw, and this was poorly formed. The shape of the foot suggests that the digits were never fully developed during ontogeny rather than lost later due to an accident or to intraspecific aggression as reported for other species of soricids ( Stephens et al. 2018).
ONTOGENY AND REPRODUCTION
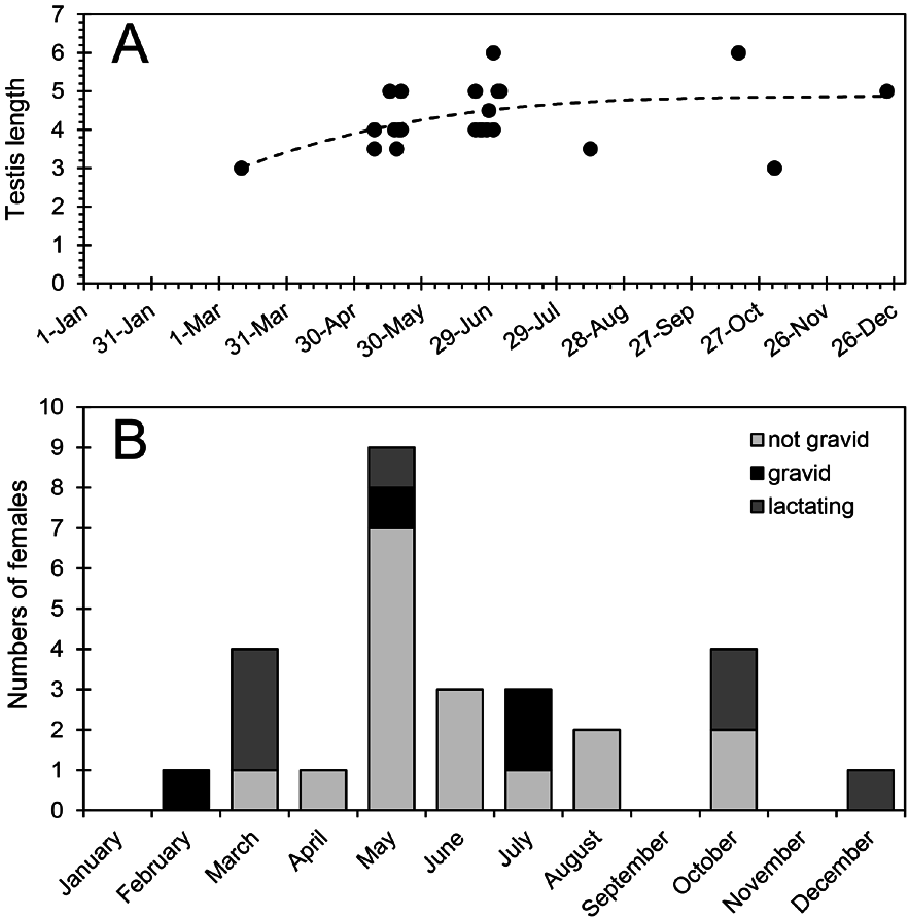
Few reproductive data are available for Cryptotis nigrescens . Gravid females have been recorded in February, May, and July ( Fig. 6 View Fig ), and a female taken on 13 October had an enlarged uterus, suggesting recent parturition. Lactating females have been recorded in March, May, October, and December. Taken together, these data suggest that reproduction occurs throughout the year rather than being synchronized with the onset of the wet season (May through December). If so, C. nigrescens is similar in this respect to other Neotropical soricids ( Woodman and Díaz de Pascual 2004; Woodman et al. 2012). Females have a total of four (two pairs) inguinal nipples. Average recorded litter size is 2, with a range of 1–3 (n = 4). This compares well with ranges of 1–2 offspring for species of Sorex and 2–3 offspring for species of Cryptotis in Guatemala and Honduras ( Woodman et al. 2012).
Average testis size of males appears to increase from March through late June or early July ( Fig. 6 View Fig ), but the significance of such an increase is difficult to determine. Most likely, it is related to the maturing of young males born earlier in the year. Woodman and Timm (1993) reported two individuals on 9 May and 18 May from Monteverde that had melanistic sheaths surrounding the testes. Both shrews had well-developed lateral glands and enlarged testes, measuring 3.5 by 2.5 mm and 4 by 2.5 mm. The significance of the melanism is unknown, particularly as the testes of soricids are inguinal throughout their lives.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cryptotis nigrescens ( Allen, 1895 )
| Woodman, Neal & Timm, Robert M 2023 |
Cryptotis parva orophila
| Choate J. R. 1970: 262 |
Cryptotis nigrescens nigrescens
| Choate J. R. 1970: 279 |
Cryptotis nigrescens zeteki
| Handley C. O. Jr. 1966: 756 |
Cryptotis tersus
| Goodwin G. G. 1954: 1 |
Cryptotis zeteki
| Setzer H. W. 1950: 299 |
Blarina (Soriciscus) nigrescens
| Allen J. A. 1895: 339 |
Blarina micrura
| Merriam C. H. 1895: 21 |
| Allen J. A. 1893: 238 |
| Tomes R. F. 1861: 279 |
| Pomel A. 1848: 249 |
| Gapper A. 1830: 202 |