Obama maculipunctata, Rossi & Amaral & Ribeiro & Cauduro & Fick & Valiati & Leal-Zanchet, 2015
|
publication ID |
https://doi.org/ 10.1080/00222933.2015.1084057 |
|
DOI |
https://doi.org/10.5281/zenodo.4324239 |
|
persistent identifier |
https://treatment.plazi.org/id/03F7B26E-FFD8-FFA0-B1A2-19C8AEC6FD7A |
|
treatment provided by |
Carolina |
|
scientific name |
Obama maculipunctata |
| status |
sp. nov. |
Obama maculipunctata View in CoL sp. nov.
( Figures 8 – 11 View Figure 8 View Figure 9 View Figure 10 View Figure 11 )
Geoplana sp. 5: Leal-Zanchet & Carbayo, 2000
Geoplana sp. 1: Carbayo et al., 2002
Geoplana sp. 2: Baptista et al., 2006
Geoplana sp. 2: Fick et al., 2006
Geoplana sp. 3: Leal-Zanchet & Baptista, 2009
Geoplana sp. 3: Leal-Zanchet et al., 2011
Etymology
The specific name refers to the dorsal pigmentation, which forms numerous flecks and dots.
Type material
Holotype. MZUSP PL.1565: leg. P. K. Boll, 10 April 2014: São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – anterior tip: transverse sections on 21 slides; anterior region at the level of the ovaries: sagittal sections on 72 slides; prepharyngeal region: transverse sections on 20 slides; pharynx: sagittal sections on 36 slides; copulatory apparatus: sagittal sections on 40 slides.
Paratypes. MZU PL .00193: leg. R. Murowaniecki , 25 November 1997: São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – anterior tip: transverse sections on six slides; anterior region at the level of the ovaries: sagittal sections on 12 slides; pre-pharyngeal region: transverse sections on 11 slides; pharynx: sagittal sections on 42 slides; copulatory apparatus: sagittal sections on 36 slides . MZU PL.00194: leg . F . Carbayo , 11 December 1997: São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – anterior tip: transverse sections on 18 slides; anterior region at the level of the ovaries: sagittal sections on 42 slides; pre-pharyngeal region: transverse sections on 15 slides; pharynx: sagittal sections on 29 slides; copulatory apparatus: sagittal sections on 34 slides . MZU PL.00195: leg . I . A . Fick , 25 November 1998: São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – pre-pharyngeal region: transverse sections on 12 slides; pharynx: sagittal sections on 36 slides; copulatory apparatus: horizontal sections on 34 slides . MZU PL.00196: leg . I . A . Fick , 23 June 2000: Praia Grande (National Park of Aparados da Serra), SC, Brazil – pre-pharyngeal region: transverse sections on 12 slides; pharynx: sagittal sections on 36 lâminas; copulatory apparatus: sagittal sections on 34 slides . MZU PL.00197: leg. S. V. Amaral , 21 March 2010: São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – anterior region preserved on clove oil; copulatory apparatus: sagittal sections on 27 slides .
Type-locality
National Forest of São Francisco de Paula, São Francisco de Paula, state of Rio Grande do Sul (RS), Brazil.
Distribution
States of Rio Grande do Sul ( São Francisco de Paula ) and Santa Catarina (Praia Grande), Brazil .
Diagnosis
Specimens of Obama with brown dorsum, sometimes greyish-brown, with a marbled appearance; live specimens with pale-yellow ventral surface in the anterior third and orange in the median and posterior thirds of the body; eyes spreading over the lateral parts of the dorsal surface; glandular margin with three types of glands; mc: h, 10 – 12%; pharynx cylindrical; prostatic vesicle with ovoid and unbranched proximal portion; penis papilla symmetrical and truncated with an almost straight ejaculatory duct; ovovitelline ducts emerging dorsally from the median third of ovaries and ascending anterior to the gonopore.
Molecular diagnosis. This species includes all populations that cluster with specimens KT 250625 View Materials to KT 250629 View Materials (see Supplementary material, Table S1), with significant support in phylogenetic analyses.
Description
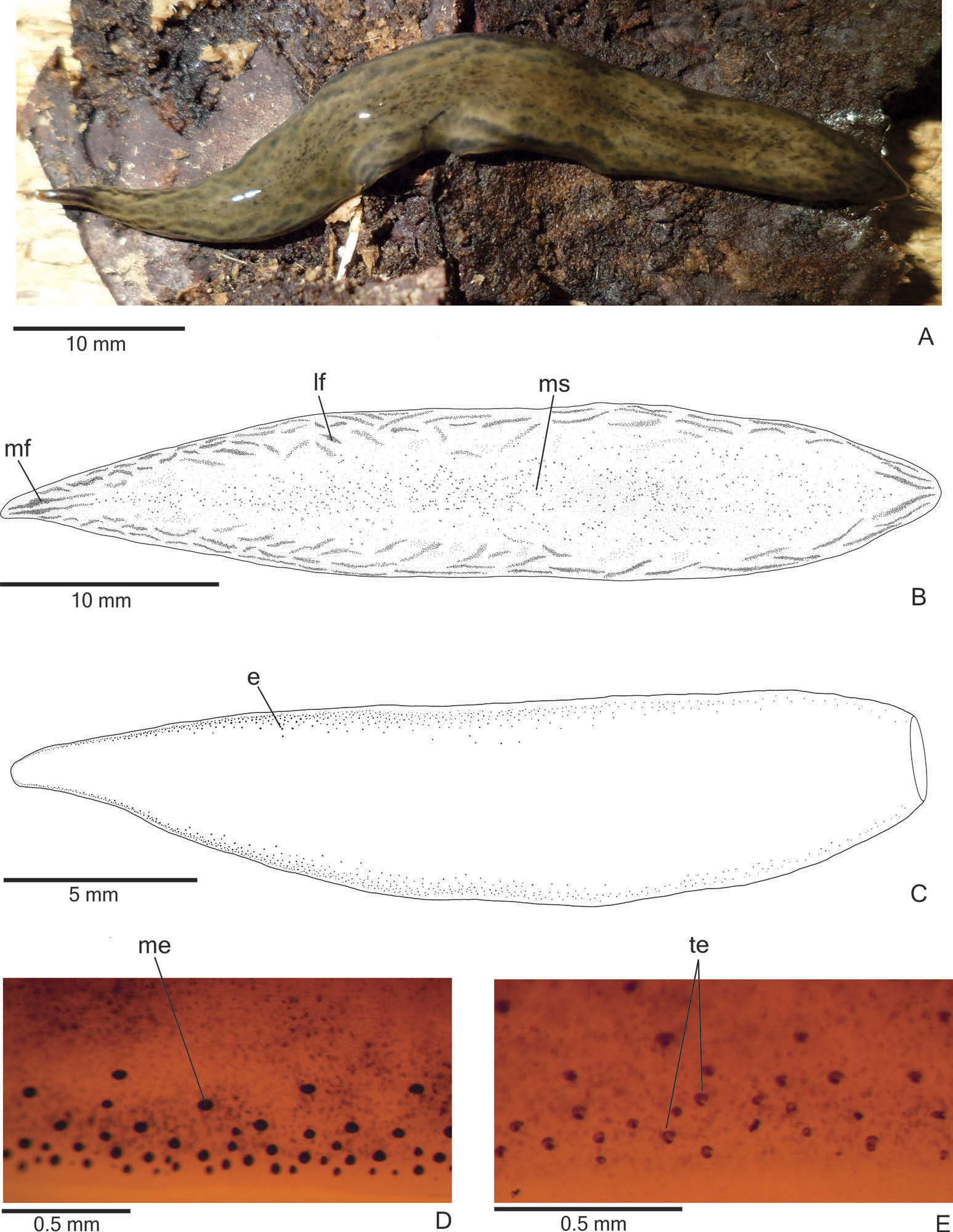
External features. Body lanceolate, elongate and flat; anterior end more narrowed than the posterior end ( Figure 8A View Figure 8 ). When crawling, maximum length reaches 72 mm ( Table 3). Mouth and gonopore located at the posterior third of the body, with the exception of the mouth in paratype MZU PL.00197, which is located at the median third of the body ( Table 3).
Live specimens with brown dorsum, sometimes greyish-brown, with a marbled appearance ( Figure 8A View Figure 8 ); ventral surface pale-yellow in the anterior third and orange in the median and posterior thirds of the body. Under moderate magnification, the anterior tip shows two median dark stripes, whereas the rest of the dorsum shows irregular greyish flecks laterally, over a light brown ground colour, and numerous dark-grey spots medially ( Figure 8B View Figure 8 ). Under the stereomicroscope, dorsal ground-colour greyish, covered by fine brownish pigmentation with dark grey spots in a wide median zone of the body and flecks on the body margins. Flecks form two median stripes on the anterior and posterior tips; these stripes are more parallel to each other anteriorly ( Figure 8B View Figure 8 ). In preserved specimens dorsal and ventral colour fade, the ventral surface becoming pale yellow.
Eyes, initially uniserial, surround anterior tip and become pluriserial immediately after this tip. After the fifth millimetre of the body, eyes spread over the lateral parts of the dorsum, forming a maximum of five longitudinal rows on either side of the dorsal surface ( Figure 8C View Figure 8 ). They are monolobated, with pigment cups of about 30 – 60 μm in diameter, on the anterior third of the body ( Figure 8D View Figure 8 ). After that, they are trilobated (with pigment cups of about 20 – 40 μm in diameter), occupying the lateral parts of the dorsal surface ( Figure 8E View Figure 8 ), but become less numerous towards posterior tip. Clear halos around eyes are absent.
Sensory organs, epidermis and body musculature. Sensory pits ( Figure 9A, B View Figure 9 ), as simple invaginations, contour anterior tip and occur ventromarginally in an irregular,
single row in the anterior seventh of the body. Initially they occur at intervals of approximately 12 µm, becoming gradually sparser. Their depth is about 25 – 40 µm.
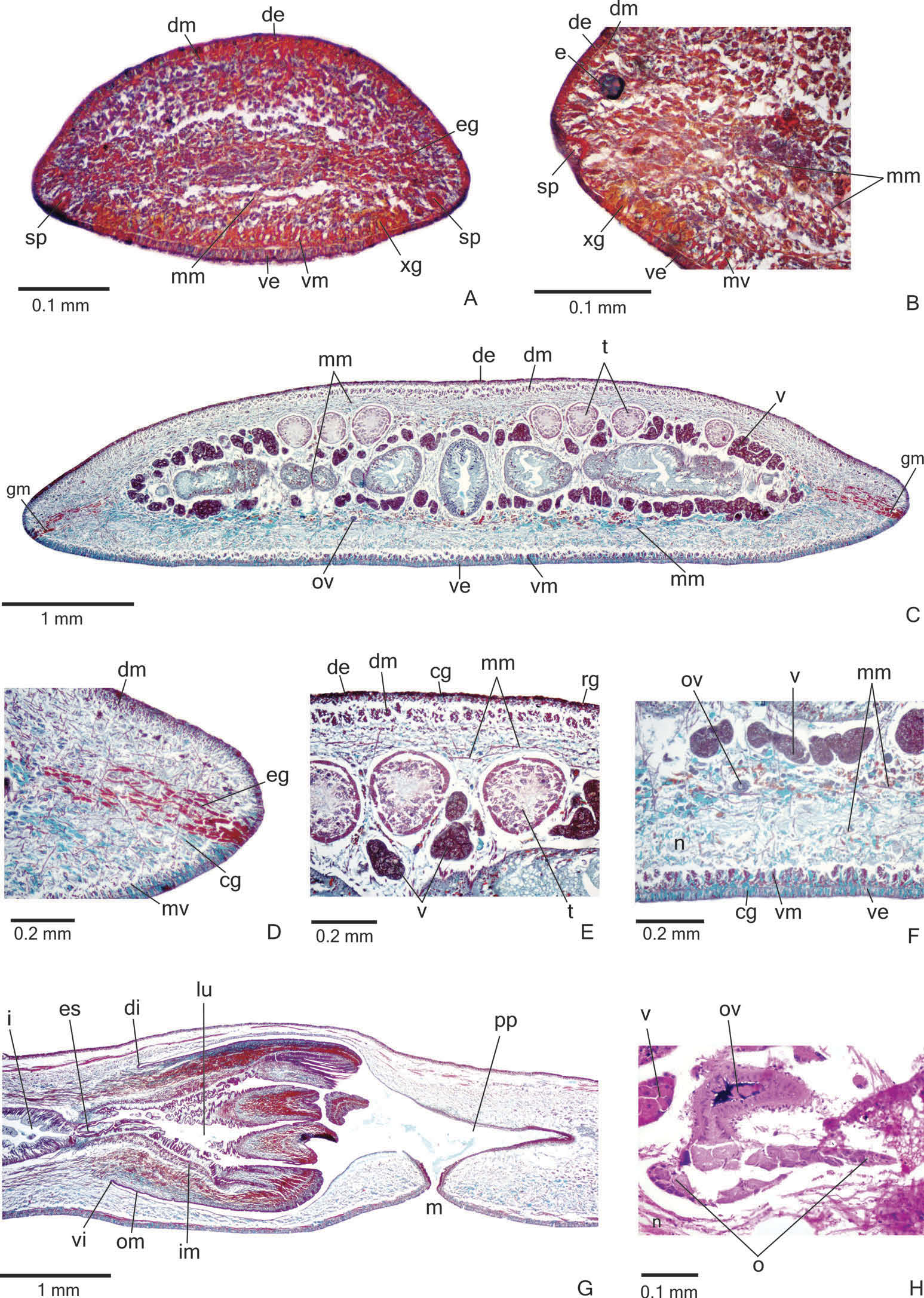
Creeping sole occupying the whole body width. Three types of glands discharge through dorsal epidermis and body margins of the pre-pharyngeal region: numerous rhabditogen cells with xanthophil secretion (rhammites) and erythrophil cells with fine granular secretion, as well as few glands with amorphous, cyanophil secretion ( Figure 9E View Figure 9 ). Creeping sole receives the secretion from numerous coarse granular, cyanophil glands ( Figure 9F View Figure 9 ), as well as few rhabditogen glands with small, xanthophil rhabdites and cells with fine granular, erythrophil secretion. After the sixth millimetre of the body, glandular margin ( Figure 9C, D View Figure 9 ) receiving openings of at least three types of glands: abundant glands with xanthophil, coarse granules, as well as scarcer erythrophil glands with fine granules and cyanophil glands with amorphous secretion. Some rhabditogen cells with small, xanthophil rhabdites open between cells of the glandular margin. On anterior tip ( Figure 9A, B View Figure 9 ), glands with cyanophil secretion and scarce glands with fine granular, erythrophil secretion open through the whole surface of the body. In addition, numerous glands with coarse granular, xanthophil secretion open through the ventral epidermis and rhabditogen glands open through the dorsal epidermis and body margins.
Cutaneous musculature with the usual three layers (circular, oblique and longitudinal layers), longitudinal layer with thick bundles ( Figure 9C – F View Figure 9 , Table 4). Cutaneous musculature thinner in the pre-pharyngeal region than in the anterior region of the body, but gradually diminishing towards anterior tip. Musculature higher medially, becoming progressively lower towards body margins; ventral musculature higher than dorsal at the sagittal plane in the pre-pharyngeal region mc: h 11 – 12% ( Table 4).
Mesenchymal musculature ( Figure 9C, E, F View Figure 9 ) mainly composed of three layers: (1) dorsal subcutaneous, located mainly close to the cutaneous musculature, with oblique fibres variously oriented (about three to four fibres thick); (2) supra-intestinal transverse (about 11 – 16 fibres thick); (3) subintestinal transverse (about seven to eight fibres thick). In addition, there are scattered ventral subcutaneous oblique fibres and transverse subneural fibres, as well as dorso-ventral fibres. On the anterior region of the body ( Figure 9A, B View Figure 9 ), the mesenchymal musculature is less developed than in the pre-pharyngeal region.
Pharynx. Pharynx cylindrical with dorsal insertion shifted posteriorly, about 6 – 7% of the body length, occupying the first two-thirds of the pharyngeal pouch; mouth in the posterior third of pharyngeal pouch ( Figure 9G View Figure 9 ). Oesophagus short; with folded wall. Oesophagus: pharynx ratio varying from 5% to 24% (10% in the holotype). It is lined by ciliated, cuboidal epithelium with insunk nuclei and coated with a thick muscle layer with circular fibres and various interposed longitudinal fibres (about 40 – 70 µm thick).
Pharynx and pharyngeal lumen lined by ciliated, cuboidal epithelium with insunk nuclei. Pharyngeal glands constituted by three secretory cell types: abundant glands with fine granular erythrophil secretion; glands with fine granular xanthophil secretion; and glands with amorphous cyanophil secretion. Cell bodies of pharyngeal glands located in the mesenchyme, mainly anterior and posterior to the pharynx.
Outer pharyngeal musculature (about 20 – 30 µm thick) comprised of thin subepithelial layer of longitudinal muscles, followed by a thicker layer of circular fibres. Inner pharyngeal musculature (about 50 – 110 µm thick) comprises a thick subepithelial layer of circular fibres, interposed with various longitudinal fibres. Outer and inner musculatures gradually become thinner towards pharyngeal tip.
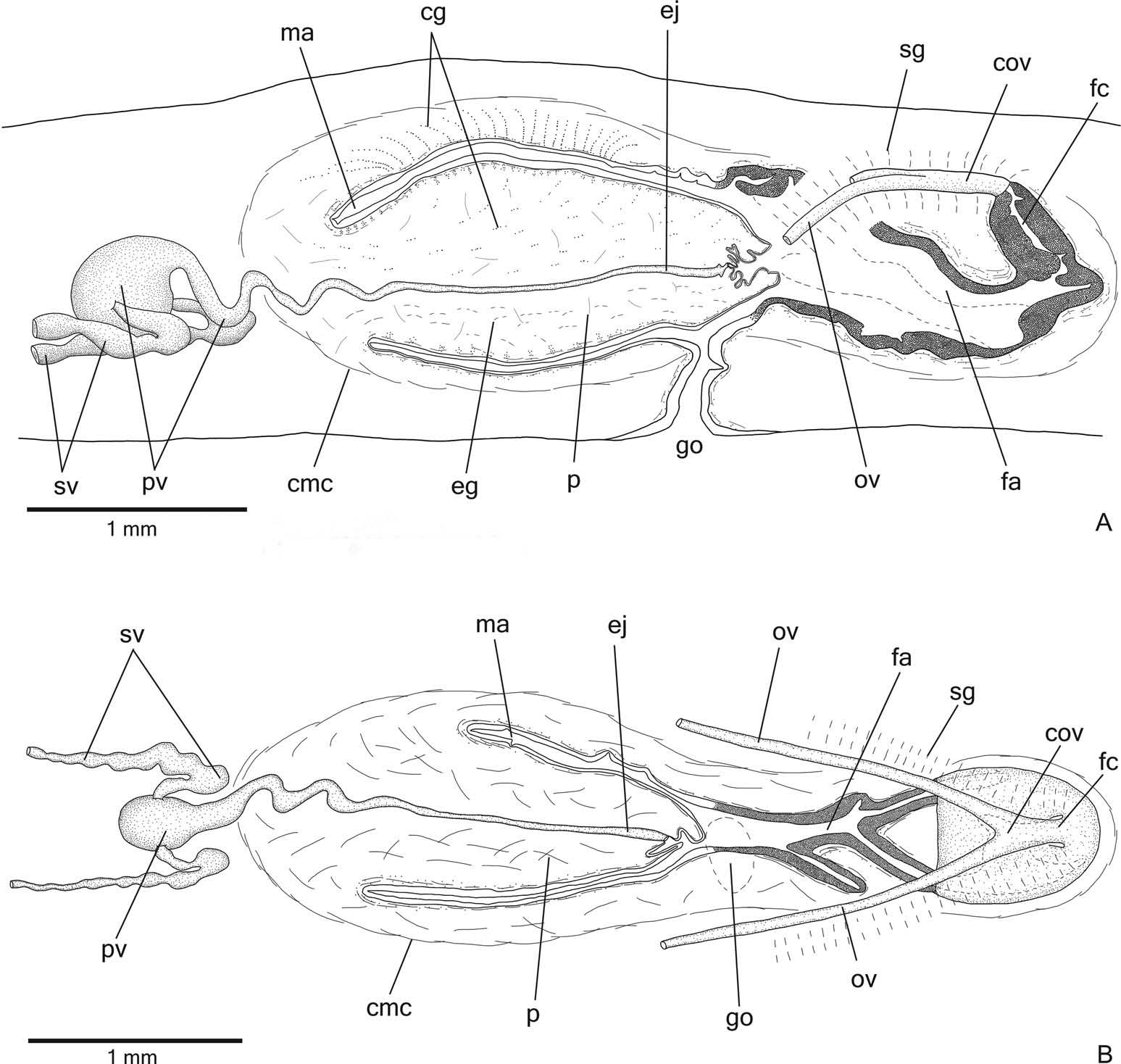
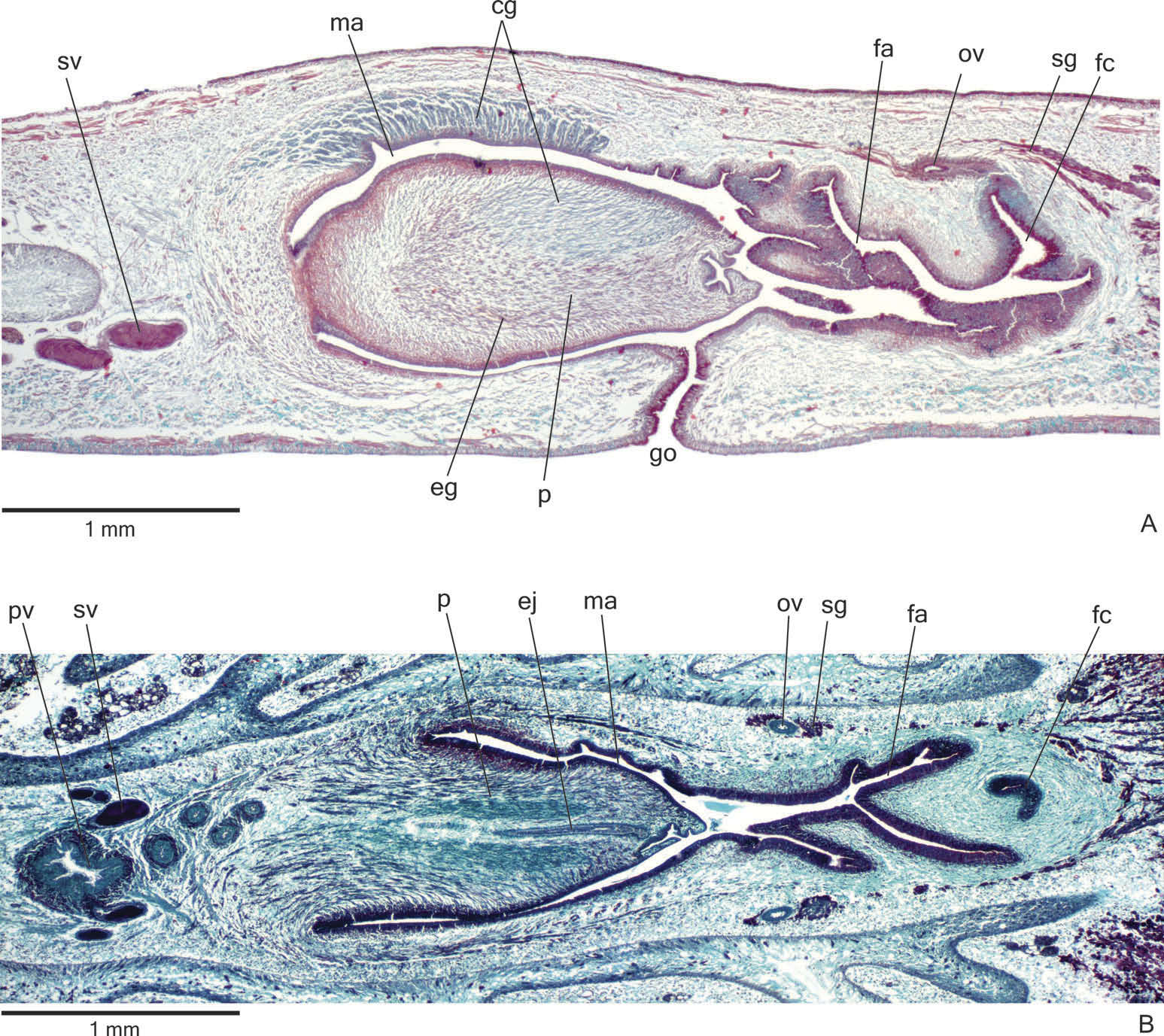
Reproductive organs. Testes in at least three irregular rows beneath the dorsal transverse mesenchymal muscles ( Figure 9C, E View Figure 9 ). They begin in the anterior third of the body and extend to near the root of the pharynx ( Table 3). Sperm ducts dorsal to ovovitelline ducts, in pre-pharyngeal region subdivided into three or four ductules. They form spermiducal vesicles posterior to the pharynx. Distally, spermiducal vesicles penetrate into the lateral wall of the proximal portion of the prostatic vesicle ( Figures 10A, B View Figure 10 , 11B View Figure 11 ). Prostatic vesicle extrabulbar, unpaired, and consisting of two portions: a globose proximal portion and a tubular, sinuous, distal portion ( Figure 10A, B View Figure 10 ). The tubular portion penetrates the common muscle coat and continues inside the penis papilla as an ejaculatory duct. Ejaculatory duct almost straight, opening at the tip of a truncated penis papilla ( Figures 10A, B View Figure 10 , 11A, B View Figure 11 ). The tip of the penis papilla may form a globose invagination with irregular contour ( Figures 10A View Figure 10 , 11A View Figure 11 ). Male atrium almost without folds, occupied by the conical and symmetrical penis papilla ( Table 3, Figures 10A, B View Figure 10 , 11A, B View Figure 11 ). The latter may occupy the distal part of the female atrium, as in paratypes MZU PL.00196 and MZU PL.00197.
Lining epithelium of sperm ducts cuboidal and ciliated; thin muscularis (about 6 – 10 µm thick) mainly constituted of circular fibres. Prostatic vesicle lined with ciliated, columnar or pseudostratified epithelium with irregular height. It receives abundant amorphous cyanophil secretions and scarcer fine granular erythrophil secretions, both from secretory cells with bodies lying in the mesenchyme, mainly around the vesicle. Muscularis of prostatic vesicle (about 50 – 120 µm thick) comprises interwoven longitudinal, oblique and circular fibres ( Figure 11B View Figure 11 ). Ejaculatory duct lined with ciliated, columnar epithelium, receiving few openings from secretory cells with weakly cyanophil, amorphous secretion. Muscle coat of ejaculatory duct thin (about 10 – 20 µm thick), constituted of interwoven circular and longitudinal fibres.
Penis papilla covered with non-ciliated, columnar epithelium, becoming lower towards the tip of the papilla ( Figure 11A, B View Figure 11 ). Penis glands of four types: cells with numerous fine granular erythrophil secretion, cells with mixed (cyanophil peripheral portion and erythrophil core), fine granular secretion and scarcer glands with cyanophil secretion and glands with xanthophil secretion. They run longitudinally in the papilla, with numerous openings through its covering epithelium, the erythrophil glands concentrating their openings at the tip and ventral surface of the penis papilla ( Figure 11A View Figure 11 ). Penis glands with cell bodies mainly external to common muscle coat and among fibres of this coat. Muscularis of the penis papilla (about 30 – 60 µm thick) composed of a thick, subepithelial layer of circular fibres interposed with some longitudinal fibres.
Epithelial lining of male atrium columnar (about 10 – 25 µm thick), non-ciliated. Three types of secretory cells empty through this epithelium: glands with fine granular, erythrophil secretion, glands with amorphous cyanophil secretion and glands with fine granular mixed secretion (cyanophil peripheral portion and erythrophil core). Numerous necks of the latter concentrate their openings at the dorsal wall of the distal portion of the male atrium ( Figure 11A View Figure 11 ). Muscularis of male atrium (about 15 – 45 µm thick) comprised of circular fibres and some interposed longitudinal fibres.
Vitellaria ( Figure 9C, E, F View Figure 9 ), situated between intestinal branches, open into the ovovitelline ducts. Ovaries ovoid or oval-elongate ( Figure 9H View Figure 9 ), measuring about 400 µm in diameter in the holotype and 200 – 300 µm in diameter in paratypes MZU PL.00194 and MZU PL.00193, respectively. They are dorsal to the ventral nerve plate, at the same transverse level as the anteriormost testes or slightly anterior to them. Ovovitelline ducts emerge dorsally ( Figure 9H View Figure 9 ) from the median third of the ovaries and run posteriorly immediately above the nerve plate. Lateral to the female atrium, ovovitelline ducts ascend postero-medially, to unite dorsal to the median or posterior third of female atrium, so forming a short common glandular ovovitelline duct. Ental portion of female atrium with an antero-dorsally directed female canal ( Figures 10A, B View Figure 10 , 11A View Figure 11 ). Female atrium ovoid with folded walls ( Figures 10A, B View Figure 10 , 11A, B View Figure 11 ). Female atrium length about half that of male atrium in the holotype, but almost the same as male atrium length in most paratypes ( Table 3).
Ovovitelline ducts and common oviduct lined with ciliated, cuboidal to columnar epithelium. They are covered with intermingled circular and longitudinal muscle fibres (about 20 – 30 μm thick). Abundant shell glands with erythrophil secretion empty into the common glandular ovovitelline duct as well as in the distal half of the ascending portion of the ovovitelline ducts ( Figures 10A, B View Figure 10 , 11A, B View Figure 11 ).
Female atrium and canal lined by tall columnar epithelium, exhibiting a multilayered aspect in some places ( Figure 11A View Figure 11 ) and with a xanthophil apical layer. Female atrium and canal receive abundant fine granular erythrophil secretion and cyanophil amorphous secretion. Cell bodies of both glands are located external to the common muscle coat or among its fibres. Muscularis of female atrium and canal (about 20 – 40 µm thick) composed of a subepithelial layer of interwoven circular and longitudinal fibres.
Gonoduct vertical at the sagittal plane. Male and female atria with ample communication, without separating folds ( Figures 10A, B View Figure 10 , 11A, B View Figure 11 ). Gonoduct lined with ciliated columnar epithelium, receiving the openings of abundant glands with fine granular erythrophil secretion and glands containing amorphous cyanophil secretion, as well as rhabditogen glands with small rhabdites. Muscularis of gonoduct consisting of a subepithelial layer of circular fibres, followed by a layer of longitudinal fibres.
Common muscle coat poorly developed, with circular, longitudinal and oblique fibres, thicker around male atrium than around female atrium. A stroma with sparse intermingled muscle fibres separates the atrial muscularis from the common muscle coat.
Comparative discussion
In general, external and internal characteristics of O. maculipunctata sp. nov. match the diagnostic characteristics of the genus Obama Carbayo et al., 2013 , so corroborating the molecular phylogenetic analyses. The new species of Obama herein described shows an internal morphology similar to four other species of this genus, namely Obama fryi ( von Graff, 1899) , Obama polyophthalma ( von Graff 1899) , Obama carinata ( Riester, 1938) and Obama eudoximariae ( Ogren & Kawakatsu, 1990) . These species share an extrabulbar prostatic vesicle with a globose proximal portion, a symmetrical penis papilla with dorsal and ventral insertions at the same transverse level, and an ovoid female atrium ( von Graff 1899; Schirch 1929; Riester 1938; Marcus 1951; Froehlich 1956; Ogren and Kawakatsu 1990).
Considering external features, by having a marbled colour pattern, the new species of Obama is similar to O. polyophthalma . It differs from O. fryi , O. carinata and O. eudoximariae , because they have homogeneous or striped patterns ( von Graff 1899; Schirch 1929; Riester 1938; Marcus 1951; Froehlich 1956). Regarding the anatomy, O. maculipunctata , which has a cylindrical pharynx, can be differentiated from O. carinata and O. eudoximariae which have a collar-type pharynx ( Schirch 1929; Riester 1938; Marcus 1951; Ogren and Kawakatsu 1990). In addition, O. maculipunctata differs from all four species by a combination of the following characteristics of the copulatory apparatus: unbranched proximal portion of the prostatic vesicle, truncated penis papilla, and almost straight ejaculatory duct traversing the penis papilla.
As a number of species of Geoplana incertae sedis have general characteristics similar to species of Obama , we have extended our comparative analysis of O. maculipunctata to this group of species. Among the 47 species indicated by Carbayo et al. (2013) as Geoplana incertae sedis, only one species, Geoplana fuhrmanni Hyman, 1962 , from Panama, shows characteristics of the copulatory apparatus similar to those of O. maculipunctata . However, having a marbled dorsum, the new species can be distinguished from G. fuhrmanni , with an almost homogeneous, spotted coloured dorsal surface, as well as by the morphology of the female atrium, which is short and funnelshaped, in contrast to the large and ovoid female atrium of O. maculipunctata .
Comparison of the new species with its congeners, using measures of intraspecific and interspecific variation of the COI gene, as well as the ABGD tool applied to the COI data set, also supported O. maculipunctata sp. nov. as a separate species. Maximum likelihood and Bayesian inference phylogenies were congruent and indicated O. maculipunctata sp. nov. as the sister group of O. carinata ( Figure 1 View Figure 1 ).
Notes on ecology and distribution
Cratera ochra sp. nov. has been recorded mainly inside and on the borders of sites with the native vegetation (mixed ombrophilous forest) of its type-locality ( Leal-Zanchet and Carbayo 2000; Carbayo et al. 2002; Leal-Zanchet et al. 2011), with a low to moderate abundance. It was also recorded in an area of dense ombrophilous forest, but not in areas of mixed ombrophilous forest, of the Research and Conservation Center Pró-Mata (Leal- Zanchet et al. 2011). Obama maculipunctata sp. nov. is abundant in areas of mixed ombrophilous forest of the Araucaria Plateau , as well as in plantations of the native Araucaria angustifolia and Pinus spp. of its type-locality ( Leal-Zanchet and Carbayo 2000; Carbayo et al. 2002). It was also recorded in areas of mixed and dense ombrophilous forest of the Aparados da Serra National Park ( Baptista et al. 2006; Fick et al. 2006), but it was not recorded in the Research and Conservation Center Pró-Mata ( Leal-Zanchet et al. 2011).
General discussion
In general, the two new species herein described match the diagnostic characteristics of their respective genera, despite their gross similarities. Species of Cratera and Obama have a similar external morphology, with a lanceolate body, usually slightly more leafshaped in species of Obama than in species of Cratera . Species of Obama can be distinguished from Cratera , as well as from other genera split off from Geoplana , by having trilobated eyes in addition to monolobated eyes ( Carbayo et al. 2013). Other anatomical features, such as the distribution pattern of the sensory pits and details of the copulatory apparatus are useful in the identification of the species. Some of these features are noteworthy especially the distribution pattern of the sensory pits and the morphology of the prostatic vesicle.
In relation to the distribution pattern of the sensory pits in both genera, Carbayo et al. (2013) indicated that members of Cratera have the sensory pits arranged in a single row on either side of the body, whereas members of Obama have two to four rows of sensory pits on either side. However, we have observed that a single irregular longitudinal row of sensory pits on either side of the body is present in O. maculipunctata . As this character may be important for distinguishing monophyletic groups, it should be further investigated in other species of Obama .
Another feature that should be analysed in more detail in species of Obama is the morphology of the prostatic vesicle. Various species have an anteriorly branched prostatic vesicle, but this feature may not have been properly assessed in earlier descriptions (cf. von Graff 1899; Schirch 1929; Riester 1938; Marcus 1951). In O. maculipunctata , the proximal portion of the prostatic vesicle is clearly unbranched.
The genus Cratera , with eight formally described species ( Carbayo et al. 2013; Rossi et al. 2014; Carbayo and Almeida 2015), had the type-species and four others molecularly analysed ( Carbayo et al. 2013). In contrast, the genus Obama , originally with 34 species, was poorly investigated at the molecular level. Four species of Obama were analysed by Carbayo et al. (2013) and, more recently, two new species were proposed for the genus based on morphological and molecular studies ( Álvares-Presas et al. 2015). It is noteworthy that one of the species included in the present phylogenetic analysis, O. carinata , showed a high intraspecific distance in relation to other species of the group. These results are also supported by the ABGD analysis, which split the specimens of O. carinata into two major mitochondrial DNA lineages, suggesting that they are two potential species. However, a Bayesian phylogenetics and phylogeography analysis ( Yang and Rannala 2010) applied for a large data set indicated that the specimens of O. carinata constituted a single species ( Álvares-Presas et al. 2015). In fact, Puillandre et al. (2012) point out that the partition output by ABGD should be considered as a first species partition hypothesis, since this result arises from the evaluation of pairwise differences of a single molecular marker. Therefore, the definition of the taxonomic status should be complemented with other evidence, such as morphological data, as done herein, in an integrative taxonomic approach ( Will et al. 2005; Padial et al. 2009; Puillandre et al. 2012).
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| PL |
Západoceské muzeum v Plzni |
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Geoplaninae |
|
Genus |