Limnochares azubi Gerecke
|
publication ID |
https://doi.org/10.5281/zenodo.170186 |
|
DOI |
https://doi.org/10.5281/zenodo.6267662 |
|
persistent identifier |
https://treatment.plazi.org/id/03F7DE69-B05A-FFFD-FEF6-FC47FCDE81A3 |
|
treatment provided by |
Plazi |
|
scientific name |
Limnochares azubi Gerecke |
| status |
sp. nov. |
Limnochares azubi Gerecke , sp. nov.
Limnochares aquatica L. (err., Crema et al. 1996)
Type series: Holotype male, SMF, France, Corsica, F 79 (2B) Ota, Rau. de Furtolaccia in Gorges de Spelunca, 250 m, 0 2.06.1993, leg. Gerecke; paratypes: SMF, same collecting site and date, 3/4/0 slide mounted, 2/6/0 undissected in Koenikes fluid; coll. Gerecke, Tübingen: F 67 Corse (2B) Calacuccia (Calvi), Fango, Rau. de Bocca Bianca, 360 m, 08°50E,42°23N, 15.05.1989 Schwoerbel et al. 0/1/0 slidemounted; F 81 Corse (2B) E visa, Rau. d'Aitone upstream Gorges de Spelunca, 300 m, 0 2.06.1993 Gerecke 1/1/0 slidemounted, 5/19/0 undissected in Koenikes fluid.
Further material examined: Italy, Mo 1 B/793 TrentinoAlto Adige (TN) Monzoni, Pozza di Fassa, Ponte della Fessura, 1660 m, 10.07.1993 leg. SAR 0/1/0 (" L. aquatica ", err., Crema et al. 1996); Spain, E 111 Andalusia (CA) Alcornocales, Rio de Los Palmones E Casas Castaño, 200 m, TF 67 21, 28.03.1994 Gerecke, 0/1/0; Morocco, Mar 3 a, 4b, Djebel Tazzeka, E Bab Ferrich, two springs, 34º03'29N, 4º10'54W, 1300 m, 0 4.04.1998, Gerecke 5/5/2.
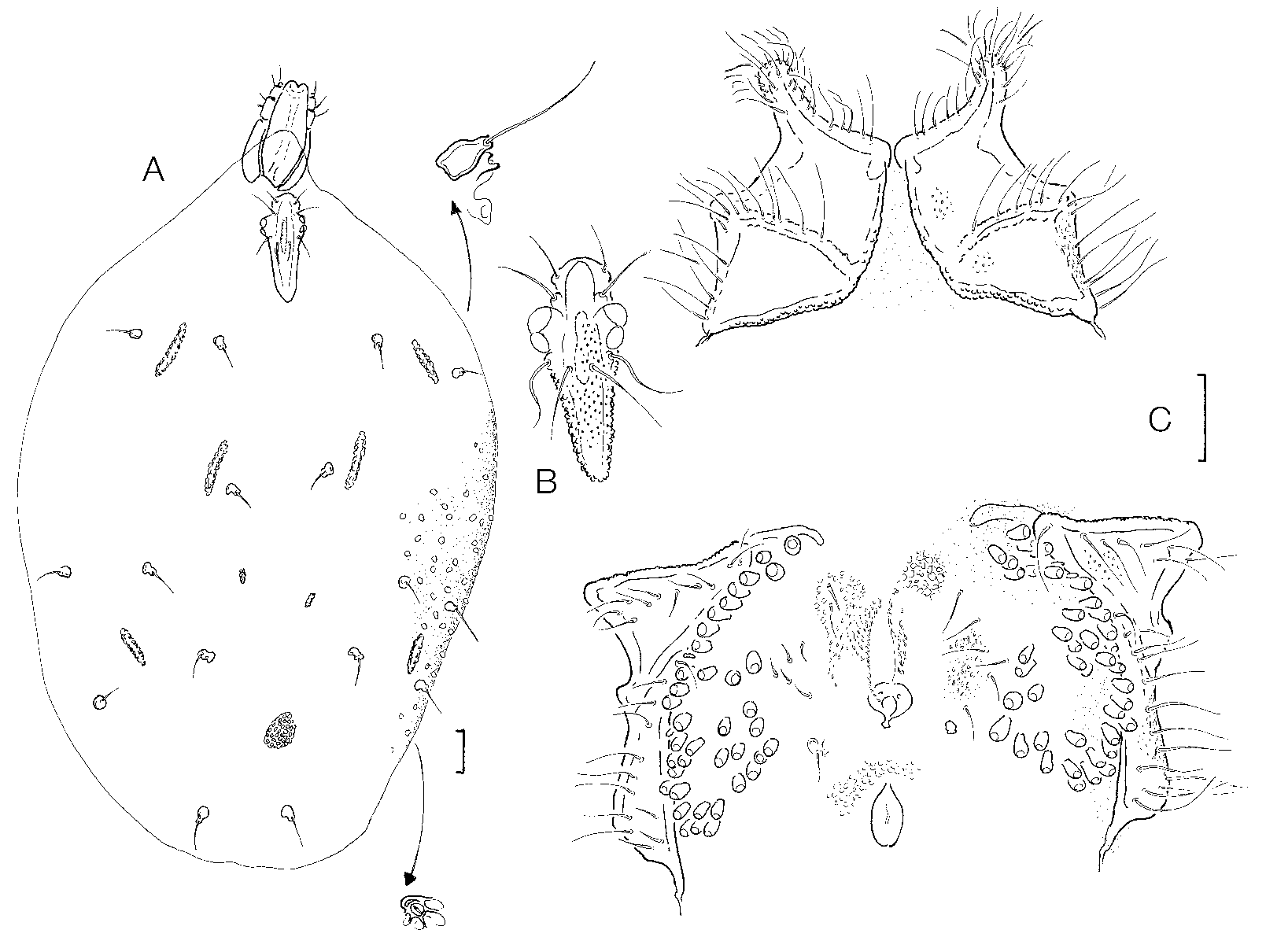
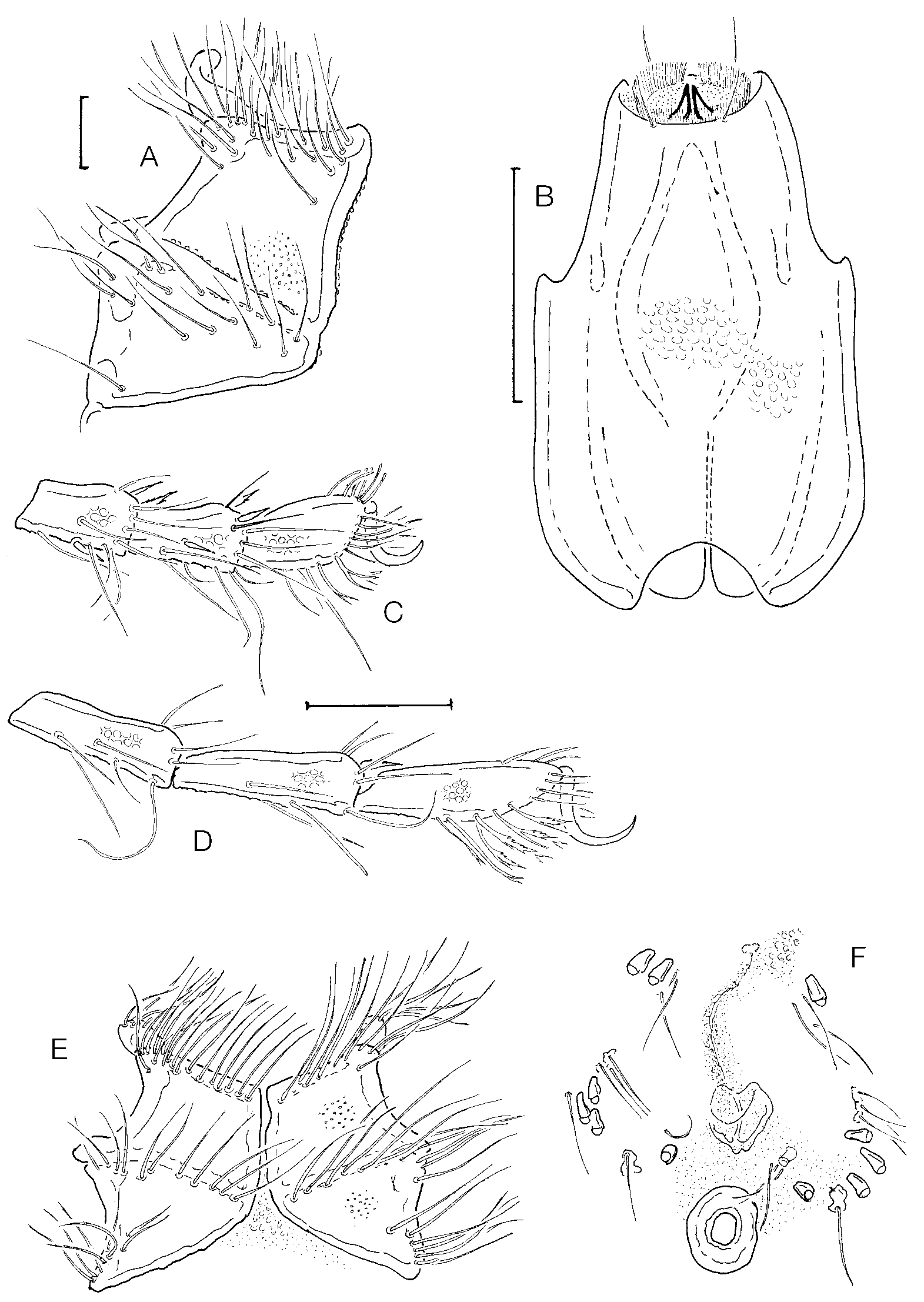
Diagnosis: Idiosoma L 900–1600, dorsum bearing sclerites ( Figs 1 View FIGURE 1 A, 2 A) at least in the anterior part, two pairs, rodshaped, and in the posterior part one pair rodshaped, one pair knobshaped and one unpaired, roundish, enlarged); frontal shield ( Fig. 1 View FIGURE 1 B) short and robust (L/H ratio 1.6–2.4); Cx1+2 ( Fig. 1 View FIGURE 1 C) slender (L/ W 1.7 –2.1), anterolateral edge with a digitiform, densely setose extension, coxal setae short; genital field ( Fig. 1 View FIGURE 1 D) with 15–80, generally <50 pairs of acetabula; genital setae in both sexes not arranged in groups; excretory pore generally smooth; palp ( Fig. 2 View FIGURE 2. A – F EF) fivesegmented, with relatively short P4 (L 14–20, L ratio P2/P 4 in males 2.0–3.6, in females 3.0–3.6).
Description: Both sexes: Integument with dense, large papillae; margins of dorsal sclerites and coxae densely covered by fine tubercles; frontal sclerite, the unpaired posterodorsal sclerite and the coxae finely porose; glandularia ( Fig. 1 View FIGURE 1 A, in top) cupshaped; frontal sclerite with rounded anterior and posterior margins, its posterior part strongly converging; setae on coxae arranged exclusively along margins and suture lines, on Cx3/4 more distanced from the sclerite borders than on Cx1/2; anterolateral corner of Cx1 forming a well developed digitiform extension with dense setation ( Fig. 1 View FIGURE 1 C); legs with a highly diversified setation, in particular at the margins of the terminal segments surrounding the claw furrow (including groups of bi or trifurcated setae, see Figs 3 View FIGURE 3. A C, D); claws simple; genital field with a long membranous gonopore flanked by a line of fine setae; anal pore generally membranous (but see discussion), close to the posterior edge of the gonopore; acetabula minute, on vaseshaped stalks, most of them arranged in a line along the medial margin of Cx3/4, the rest grouped in the area between Cx4 and the genital setae; gnathosoma elongated, its base in ventral view ( Fig. 1 View FIGURE 1 A) with nearly parallel margins, in lateral view ( Fig. 2 C View FIGURE 2. A – F ) with equally convex ventral margin, rostrum large, bent ventrally; chelicerae ( Fig. 2 D View FIGURE 2. A – F ) long and with minute, but strong claws; palp ( Fig. 2 View FIGURE 2. A – F EF) fivesegmented, with several long, hairlike setae; P4 subquadrate, P5 fine and slender, bearing a pair of apical setae.
Males (n = 7): Idiosoma L 1000–1600; frontal sclerite L/W 198–288/100–153; anterodorsal sclerites L 90–126/ 116–166; Cx1 maximum L/anterior margin W 265–315/ 139–180; Cx3/4 maximum L/anterior W (excluding the subcutaneous apodemes) 324–440/153–207; leg measurements (L/H) of one paratype male:
Segment IL IIL IIIL IVL
1 43/56 49/61 45/54 40/58
2 61/52 69/49 52/36 83/43
3 76/49 78/45 78/38 100/38
4 78/45 83/40 90/36 121/38
5 76/47 83/40 99/38 123/38
6 96/51 110/45 123/36 141/38
Acetabula number 38–78, genital setae (number 14–32) arranged in an irregular line parallel to the gonopore; genital skeleton minute, in shape as described for L. aquatica by Barr (1972); gnathosoma L 247–312, H 148, W 157–171, mouth disc diameter 52–67; chelicera basal segment L/H 183–260/34–56, claw L 22–27 (L/H ratio 5.1–6.1, basal segment/claw L ratio 7.6–9.6); palp segments L/H P 1 9–14/20 –29, P2 43–54/30–33, P 3 29–35 /26–29, P 4 14–17/15 –17, P 5 18–23 /8, total L 113–140.
Females (n = 7): Idiosoma L 900–1600; frontal sclerite L/W 234–351/130–202; anterodorsal sclerites L 85–224/100–270; Cx1 maximum L/anterior margin W 275–460/ 139–234; Cx3/4 maximum L/anterior W (excluding the subcutaneous apodemes) 324–535/135–310; Ac number 30–160, genital setae number 8–28; eggs, maximum number 30, maximum diameter 150; gnathosoma L 247–333, H 123–157, W 166, mouth disc diameter 56–76; chelicera (n = 1) basal segment L/H 172/40, claw L 27 (L/H ratio 5.0, basal segment/claw L ratio 6.4); palp segments L/H P 1 9–17/21 –31, P2 38–59/ 32–40, P3 33–39/30–35, P 4 16–23/19 –25, P 5 21–23 /8–9, total L 122–156.
Deutonymphs (material from Morocco): Idiosoma L 1100, frontal shield L/W 207/ 103, dorsal sclerites 1, 2 L 45, 67, Cx1/2 L/W 216/112, Cx3/4 247/117; coxae with a few setae only, but considerably differing between the two specimens investigated in number and arrangement (in specimen from site Mar 4 b Cx1+2 n = 30, many of them in a dense group on the anterolateral projection, Cx3+4 n = 15; in specimen from site Mar 3 a Cx 1+2 n = 15, absent from the anterolateral projection; Cx3+4 n = 4); acetabula number 40, only one genital seta; gnathosoma L 206, mouth disc radius 49, chelicera basal segment L/ H 145/34, claw L 20; palp measurements P 1 7/19, P 2 31–35 /29–32, P 3 23–25/25 –28, P 4 12–13/14 –15, P 5 16/7, total L 91.
Dervivatio nominis: " azubi " is an undeclineable fantasy name.
Discussion: From a comparison of important measurements of 16 specimens from all collecting areas no remarkable differences were found between populations from Morocco, Spain, Corsica and the southern Alps. An analysis of specimens from the locus typicus, showed L. azubi to be a species with a high degree of variability in many characters. One female, taken together with numerous typical specimens in a small Moroccan mountain spring, merits particular attention (and would have been described by the author as a new species if it had been found as a single specimen). It differs from all other females not only in having a larger frontal shield (L 351, others <290) and larger coxae (L Cx1+2/3+4 460/535, others <350/440), but also in number of acetabula (155, others <90) and genital setae (28, others <15). Furthermore, this female has an increased number of muscle attachment sclerites (including one asymmetrically arranged on the level of the posterodorsal sclerite), and its anal pore is surrounded by a large sclerite ring. Otherwise, with regard to measurements of the gnathosoma and its appendages, it fits completely the variability range of the population from the locus typicus. At our present state of knowledge, it is impossible to take a definitive decision about the taxonomic state of this specimen. As the sclerotization of the anal pore is generally a stable speciesspecific character, it could be supposed to represent a separate, still undescribed species. An alternative hypothesis postulated is that L. azubi , as has been described for L. aquatica ( Böttger 1972) , is capable of supernumerary moults at the adult stage. Under these conditions, the particular idiosoma morphology of the specimen in question could be explained as the consequence of a morphological change taking place when adults of L. azubi moult. Possibly, additional moults allow for a subsequent increase of idiosoma volume and a correspondingly higher egg production in females. The appearance of additional sclerites corresponds to the need to resist water flow, a need which increases with growing body volume ( Mitchell 1958). The fact that mouth parts are not involved in this reorganization could be easily explained if they were adapted to the uptake of prey of a defined size. Also regarding the deutonymphs, the considerable differences suggest that the two specimens represent two different moulting stages.
Within the subfamily Limnocharinae, the presence of muscle attachment plates usually is found combined with a reduction in the number of palp segments (all species belonging to the genus Neolimnochares , distributed in America, Asia, Australia and Africa). The only known species with a fivesegmented palp and sclerotized dorsal muscle attachments is L. anomala Habeeb, 1965 from California, described from a single deutonymph. Adults of that species have since been found and D.R. Cook kindly made available unpublished data from his investigations (Cook unpubl.). Limnochares azubi agrees with L. anomala in the arrangement of muscle attachment sclerites, shape of the frontal shield and gnathosoma and the number and arrangement of elements of the genital area. The most conspicuous difference is the presence of a prominent, subquadratic sclerite with tubercular projections surrounding the anal pore (in L. azubi , the anal pore is generally unsclerotized or in exceptional cases with a circular sclerite ring not bearing tubercles). Furthermore, L. anomala has no anteriolateral extension on Cx1 (as in L. aquatica only a subcutaneous apodeme is found here), and a relatively longer P4 (P4/P5 equal in L). The presence of two sister species from such separate zoogeographic areas suggests they are of ancient origin. The phylogenetic relations between limnocharid species and genera should be reconsidered.
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |