Myrsidea philydori, Kolencik & Sychra & Papousek & Kuabara & Valim & Literak, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4418.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:04FEA195-71DA-4C7E-A62B-A658CFCF6B0C |
|
DOI |
https://doi.org/10.5281/zenodo.6487985 |
|
persistent identifier |
https://treatment.plazi.org/id/03F887AB-FFE1-FFC8-A5F7-D5B7FCB9188C |
|
treatment provided by |
Plazi |
|
scientific name |
Myrsidea philydori |
| status |
sp. nov. |
Myrsidea philydori , new species
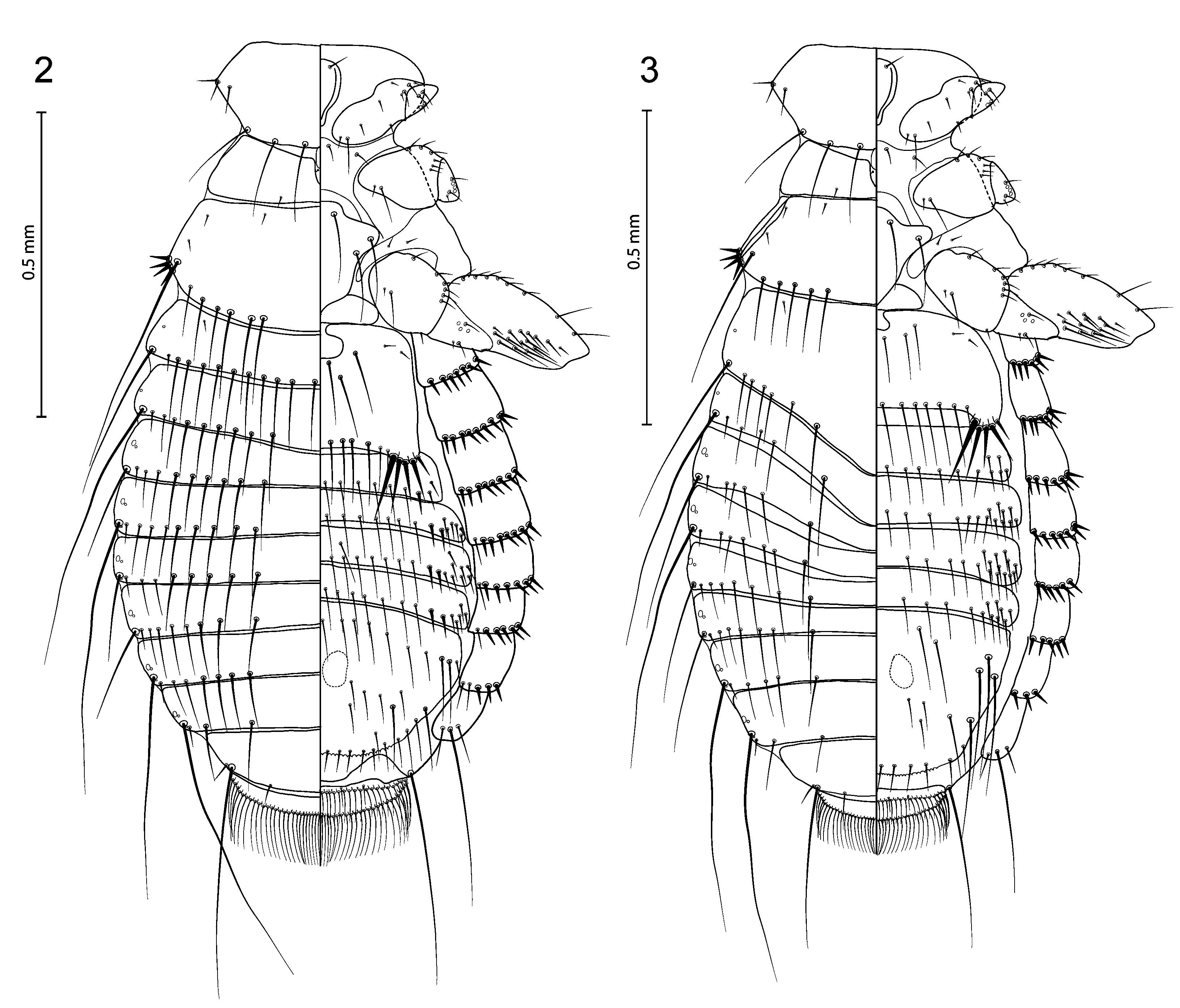
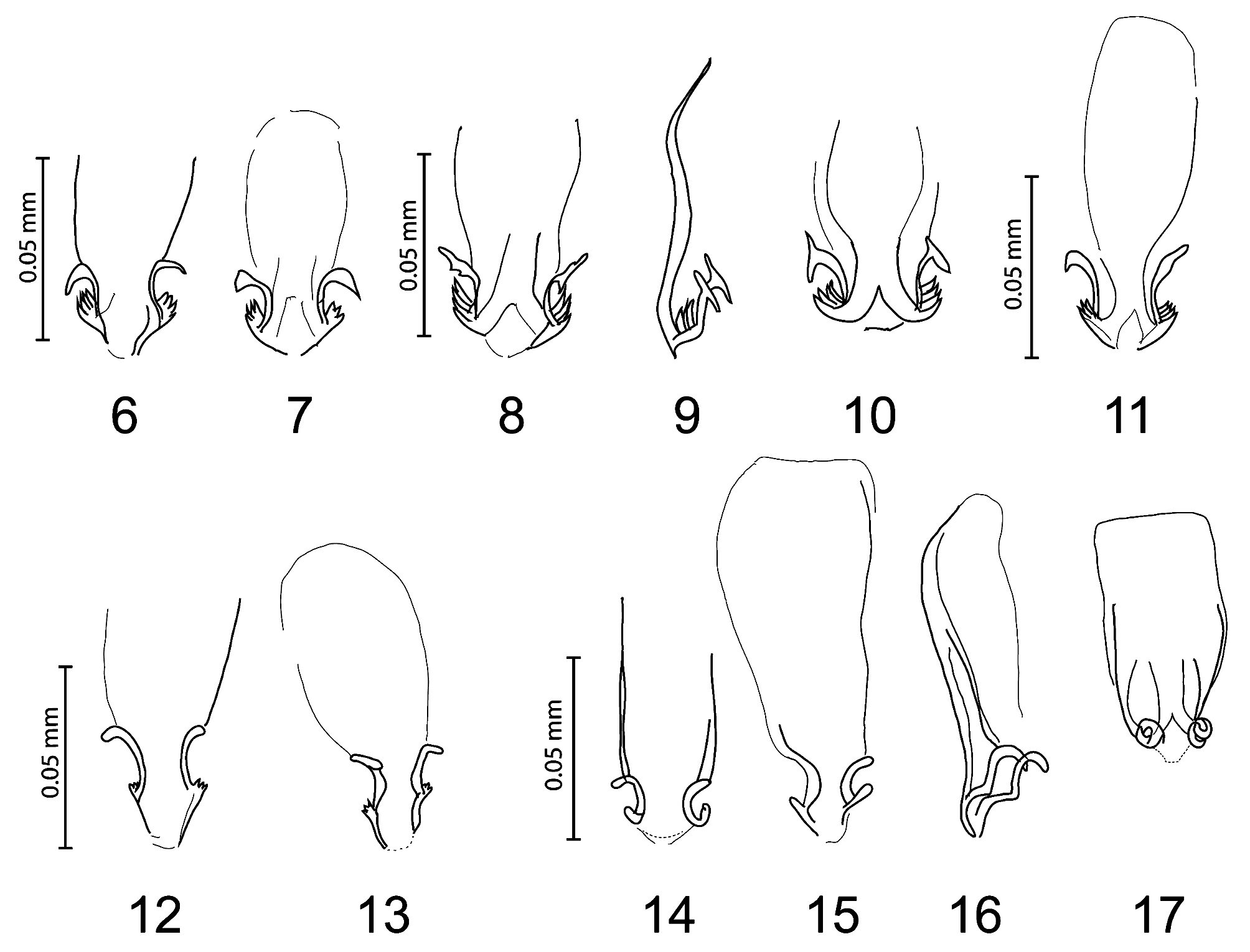
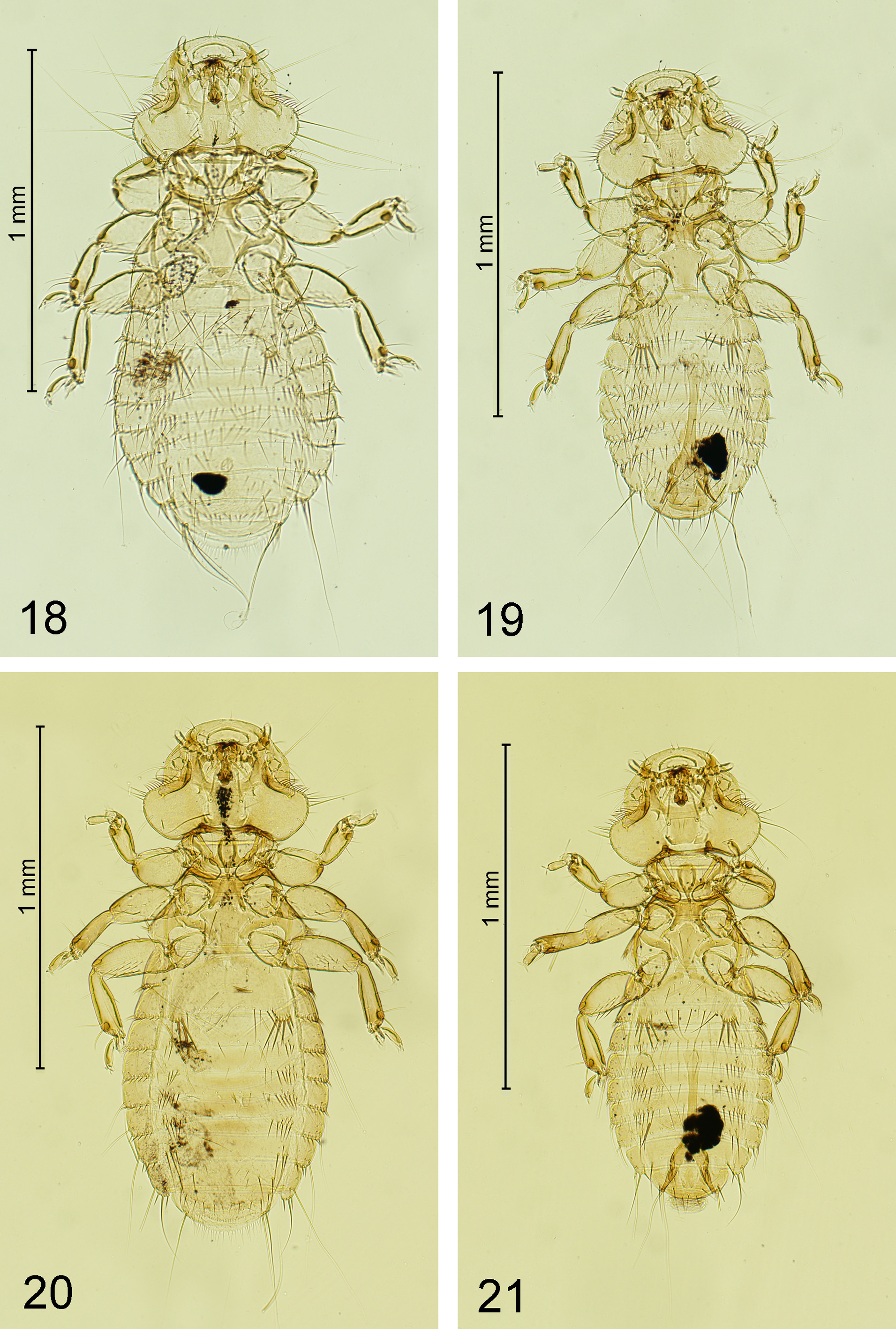
( Figs 3 View FIGURES 2–3 , 8–10 View FIGURES6–17 , 20–21 View FIGURES 18–21 )
Type host. Philydor rufum (Vieillot, 1818) —buff-fronted foliage-gleaner.
Type locality. San Rafael National Park , Paraguay (26°30'S, 55°47'W). GoogleMaps
Type material. Ex Philydor rufum : holotype ♀, San Rafael National Park, Paraguay (26°30'S, 55°47'W), 19 August 2012, I. Literak ( MMBC) GoogleMaps . Paratypes: 2♀, 3♂, with the same data as holotype GoogleMaps .
Other material, non-type. Ex Anabacerthia lichtensteini (Cabanis & Heine, 1859) —ochre-breasted foliagegleaner: 1♂, San Rafael National Park, Paraguay (26°30'S, 55°47'W), 20 August 2012, I. Literak ( MMBC). GoogleMaps
Diagnosis. Myrsidea philydori n. sp. shares the same type of male genital sac sclerite as other Myrsidea from members of the Furnariidae . According to the shape of female tergites, M. philydori is close to M. waterstoni Valim, Price & Johnson, 2011 from Anabacerthia variegaticeps (P.L. Sclater, 1857) from Panamá. Female of M. philydori can be easily distinguished from those of M. waterstoni by the absence of a detached plate on tergite III and a larger number of setae on tergites IV–VI (12–14 vs 7–10 respectively). Males of M. philydori and of M. waterstoni are very similar in setal counts, but they can be separated by smaller dimensions in all measurements, especially TW (0.42–0.44 vs 0.47) and PW (0.26–0.28 vs 0.31). Comparing M. philydori with other Neotropical Myrsidea , the new species has modified tergites similar to those of M. rekasii Dalgleish & Price, 2003 and M. baileyae Dalgleish & Price, 2003 , both described from the Pipridae . However, males of these latter species have completely different type of genital sac sclerite. Females of M. philydori differ from those of M. rekasii by smaller numbers of setae on tergites I–III (9–10 vs 12–14 on I; 6–8 vs 11–18 on II; 10–11 vs 13–16 on III) and from those of M. baileyae by smaller number of setae on tergite VIII (4 vs 8–9), as well as by smaller dimensions, especially TW (0.47–0.48 vs 0.54–0.55).
Description. Female (n = 3). As in Figs 3 View FIGURES 2–3 and 20 View FIGURES 18–21 . Hypopharyngeal sclerites fully developed. Length of dhs 10, 0.051–0.055; dhs 11, 0.098–0.111; ratio dhs 10/11, 0.46–0.55; ls5 0.04 long, latero-ventral fringe with 10–11 setae. Gula with 4 setae on each side. Pronotum with 6 setae on posterior margin and 3 short spiniform setae at each lateral corner. First tibia with 3 outer ventro-lateral and 4–5 dorso-lateral setae. Metanotum not enlarged, with 8–11 marginal setae; metasternal plate with 4 setae; metapleurites with 4 short strong spiniform setae. Femur III with 13–15 setae in ventral setal brush. Tergites modified as follows: I–II strongly convex, III–IV are depressed by I–II ( Fig. 3 View FIGURES 2–3 ). Abdominal segments with well-defined median gap in each row of tergal setae. Tergal setae: I, 9–10; II, 6– 8; III, 10–11; IV, 12–13; V, 12–14; VI, 12–14; VII, 7–8; VIII, 4; Postspiracular setae very long on II, IV, VII and VIII (0.35–0.46); long on I and III (0.23–0.32); and short on V and VI (0.13–0.20). Inner posterior seta of last tergum not longer than anal fringe setae with length 0.03–0.06; length of short lateral marginal seta of last segment, 0.03–0.06. Pleural setae: I, 6–7; II, 7–8; III, 7–8; IV, 6–7; V, 5–6; VI, 5–6; VII, 3–4; VIII, 3. Pleurites without slender and longer setae. Pleurite VIII with inner setae (0.02–0.04) as long as outer (0.02–0.04). Anterior margin of sternal plate II with a medial notch. Sternal setae: I, 0; II, 5 in each aster: s1, 0.10–0.12; s2, 0.09–0.10; s3, 0.07– 0.08; s4, 0.05–0.06; s5, 0.04–0.05; with 13–14 marginal setae between asters, 6 medioanterior; III, 20–23; IV, 29– 34; V, 31–37; VI, 25–28; VII, 12–15; VIII–IX, 11–12; and 11–13 setae on deeply serrated vulvar margin; sternites III–VII without medioanterior setae. Anal fringe formed by 38–40 dorsal and 33–43 ventral setae. Dimensions: TW, 0.47–0.48; POW, 0.36–0.37; HL, 0.32–0.33; PW, 0.29; MW, 0.46–0.48; AWIV, 0.60; ANW, 0.20–0.25; TL, 1.47–1.55.
Male (n = 4). As in Fig. 21 View FIGURES 18–21 . Similar to female except as follows: length of dhs 10, 0.048–0.054; dhs 11, 0.092– 0.100; ratio dhs 10/11, 0.48–0.59; ls5 0.03 long, latero-ventral fringe with 10–11 setae. Gula with 3–4 setae on each side. Metanotum not enlarged with 4–7 marginal setae; metasternal plate with 4 (6 in specimen from A. lichtensteini ) setae; metapleurites with 3–4 short spiniform strong setae. Femur III with 10–14 setae in ventral setal brush. Abdominal segments with well-defined median gap in each row of tergal setae. Tergal setae: I, 7–9; II, 8–11; III–IV, 9–11; V, 11–12; VI, 9–11; VII, 4–6; VIII, 4; Postspiracular setae with the same pattern as in female but shorter. Length of inner posterior seta of last tergum, 0.05; short lateral marginal seta of last segment, 0.02. Pleural setae: I, 5–6; II, 6–7; III, 6–7; IV, 6; V, 5; VI, 4–5; VII, 3; VIII, 3. Pleurite VIII with inner setae (0.03–0.04) as long as outer (0.02–0.03). Anterior margin of sternal plate II with a medial notch. Sternal setae: I, 0; II, 4–5 in each aster: s1, 0.09–0.12; s2, 0.07–0.10; s3, 0.06–0.07; s4, 0.04–0.05; s5, 0.02–0.04; with 12–14 marginal setae between asters, 4–6 medioanterior; III, 16–23; IV, 24–28; V, 22–33; VI, 20–26; VII, 10–14; VIII, 4; remainder of plate, 8– 10; and with 3–4 setae posteriorly; sternites III–VII without medioanterior setae. With 8 internal anal setae. Genital sac sclerite as in Figs 8–10 View FIGURES6–17 . Dimensions: TW, 0.42–0.44; POW, 0.33–0.35; HL, 0.31; PW, 0.26–0.28; MW, 0.37– 0.39; AWIV, 0.47–0.48; GW, 0.11–0.12; GSL, 0.08; TL, 1.23–1.33.
Etymology. The species epithet is a noun in apposition derived from the generic name of the type host.
Remarks. These are first records of chewing lice from both Philydor rufum and Anabacerthia lichtensteini . A portion of COI gene was sequenced from one specimen of M. philydori from Anabacerthia lichtensteini from Paraguay (GenBank MF563530 View Materials ). Comparing our sequence with other known sequences of Neotropical Myrsidea , divergences exceeded 19% in all cases, including that with M. waterstoni (ex Anabacerthia variegaticeps , family Furnariidae, GenBank FJ 171278 View Materials ) being 19.3%. Curiously, comparing our sequence of M. philydori with all known Myrsidea sequences, the closest was that of M. textoris Klockenhoff, 1984 (ex Ploceus intermedius Rüppell, 1845 and Ploceus velatus Vieillot, 1819 , family Ploceidae, GenBank KF768813 View Materials and KF768815 View Materials ) from South Africa, with a p-distance of about 17.5%. Furthermore, sequences of a portion of EF-1alpha gene of M. philydori from Philydor rufum and from Anabacerthia lichtensteini (GenBank MF574203 View Materials – MF574204 View Materials ) were identical to each other, and diverged from that of M. waterstoni (GenBank FJ171305 View Materials ) by 3.4%. All these sequence divergences are large enough to confirm M. philydori as a new, separate species.
| MMBC |
Moravske Muzeum [Moravian Museum] |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |