Raphidascaris (Sprentascaris) saltaensis, Ailán-Choke, Lorena Gisela, Ramallo, Geraldine & Davies, Dora, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4231.1.10 |
|
publication LSID |
lsid:zoobank.org:pub:8D663345-A7C6-4249-86E2-C79FE6D985C6 |
|
DOI |
https://doi.org/10.5281/zenodo.5689383 |
|
persistent identifier |
https://treatment.plazi.org/id/03F987B3-6602-8E69-54D7-FC756FAFFC69 |
|
treatment provided by |
Plazi |
|
scientific name |
Raphidascaris (Sprentascaris) saltaensis |
| status |
sp. nov. |
Raphidascaris (Sprentascaris) saltaensis View in CoL sp. nov.
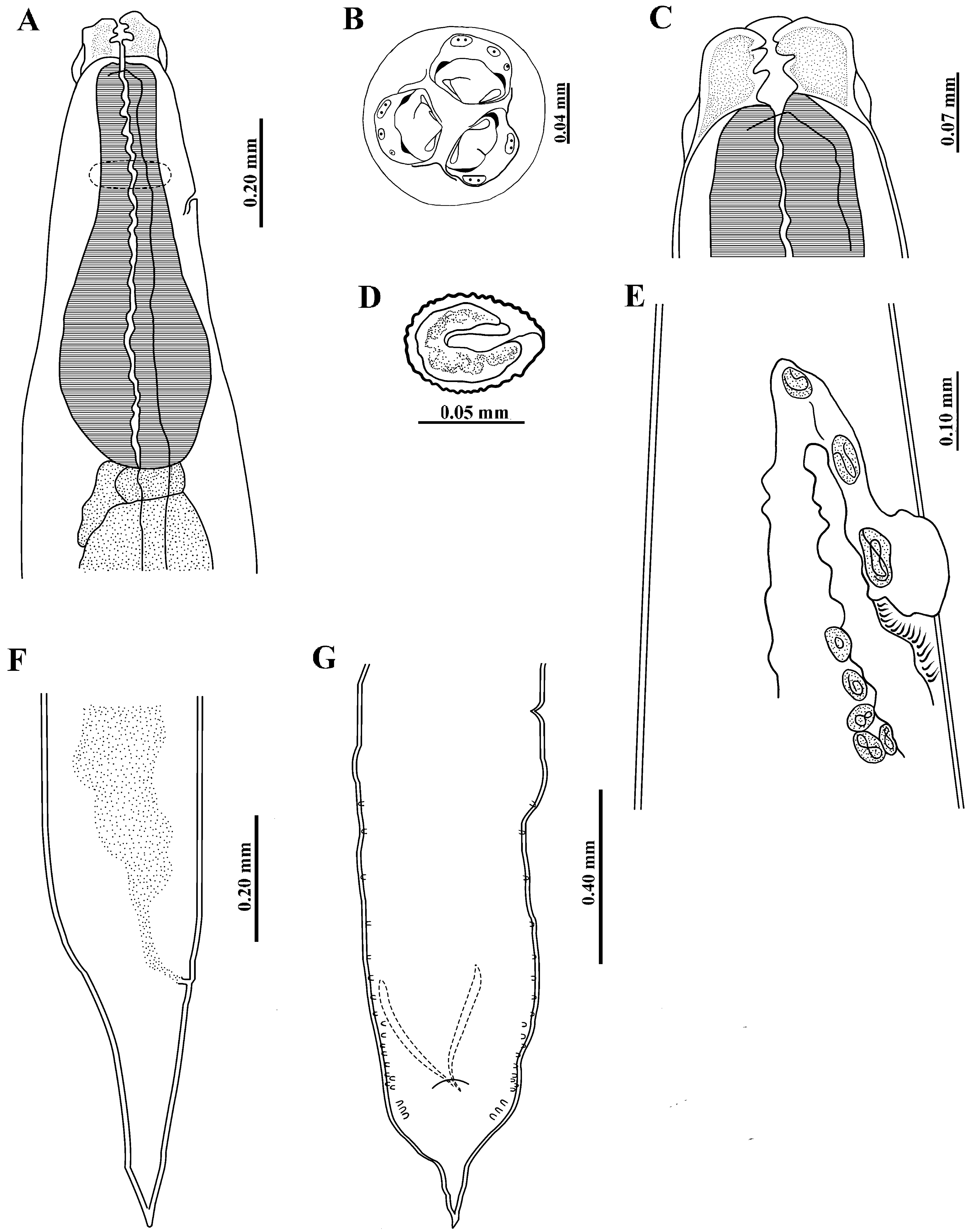
( Figs 1–2 View FIGURE 1 View FIGURE 2 )
Type material. Holotype: female CH-FML # 07663; paratypes: eight males and five females, of which nine specimens were used for SEM, CH-FML # 0 7665.
Type host. Rineloricaria steinbachi Regan , ( Siluriformes : Loricariidae ) (IBIGEO-I # 355–422).
Type locality. La Caldera River (24°35´40.07”S; 65°22´11”W), La Caldera Department, province of Salta, Northwest Argentina. GoogleMaps
Etymology. The new species is named for its geographical location in the province of Salta.
Site of infection. Intestine.
Infection parameters. Prevalence: 11.94% (8/67); media intensity: 2.63 per fish.
Measurements. Table 1 View TABLE 1 .
General description. Medium sized nematodes. Cuticle bearing transverse striations. Mouth aperture triangular, two ventrolateral lips and one dorsal lip. Lips well-developed and oval-shaped; dorsal lip slightly smaller than the two ventrolateral ones. Dorsal lip bearing two double papillae, ventrolateral lips with one double papilla, one single papilla and one amphid. Lips with lateral membranous margins forming finger-shaped protrusions at each side. Membranous extensions at base of lips. Interlabia absent ( Figs. 1 View FIGURE 1 A–C; 2A–C). Excretory pore slightly posterior to nerve ring level. Lateral alae present in both sexes, beginning in lateral region below lips and reaching almost to tail tip. Muscular oesophagus short with expanded posterior half. Caudal alae absent in both sexes. Ventriculus shorter than wide, ventricular appendix longer than wide. Both sexes with conical tail, its distal tip slender and sharply pointed.
Adult males (eight specimens): Spicules equal, simple with pointed distal tips. Twenty pairs of caudal papillae. Preanal papillae: Sixteen subventral pairs; counting from cloaca opening, the first until the seventh pair are closer to each other than the remaining pairs. Adanal papillae: only one subventral pair. Postanal papillae: three small subventral pairs. Lateral alae present, without the partition in the caudal region. Caudal alae absent. Conical tail ( Fig. 1 View FIGURE 1 G).
Adult female (six specimens): Vulva preequatorial, posterior to oesophagus end level ( Fig. 1 View FIGURE 1 E). Eggs ovalshaped, with thick and rough shell provided with membranous striations, mature eggs larvated ( Figs. 1 View FIGURE 1 D). Conical tail ( Figs. 1 View FIGURE 1 F; 2D).
Remarks. Raphidascaris (Sprentascaris) saltaensis sp. nov. is distinguished from their congeners, by possessing 16 pairs of preanal papillae, membranous elevations below the lips, mature eggs with striated shell and the size of spicules (0.24 mm). For more detailed description of morphometric differences between the new species and other species of Raphidascaris (Sprentascaris) see Table 2.
Raphidascaris (S.) saltaensis sp. nov. differs from R. (S.) mahnerti and R. (S.) lanfrediae by the absence of caudal alae in the males; but shares this feature with R. (S.) pimelodi , R. (S.) hypostomi and R. (S.) marano . The new species presents lateral alae, which begins in the base of ventrolateral lips and extends almost to tail tip; no partition of this structure is observed, hence caudal alae are not present. Raphidascaris (S.) saltaensis sp. nov differs from R. (S.) pimelodi and R. (S.) hypostomi by the presence of lateral alae, and by the number of postanal papillae pairs; R. (S.) saltaensis possess three pairs, while R. (S.) pimelodi and R. (S.) hypostomi present five pairs and lack lateral alae ( Moravec 1998).
Raphidascaris (S.) saltaensis sp. nov. resembles R. (S.) marano , both species have lateral alae and three pairs of postanal papillae; but can readily be distinguished by the number of preanal papillae (16 vs. 22), the shape and size of lips (smaller and different lips with protrusions vs. longer, symmetrical, simple and equal lips), shape of eggs (rough-shelled vs. thin-shelled) and the spicule length (0.24 vs. 0.27) ( Moravec 1998; Ramallo 2009).
Discussion. Five genera of Anisakidae have been reported from the intestine of Neotropical freshwater fishes: Goezia (Zeder, 1800) , Hysterothylacium (Ward and Magath, 1917) , Terranova (Leiper and Atkinson, 1914) , Raphidascaroides (Yamaguti, 1941) and Raphidascaris (Railliet and Henry, 1915) . Goezia , Hysterothylacium and Raphidascaris show a broad geographical distribution including Mexico, Guyana, Venezuela, Brazil, Paraguay, Peru and Argentina; in contrast Raphidascaroides and Terranova were only recorded in Brazil and Venezuela ( Moravec 1998). Raphidascaris has two subgenera: Raphidascaris (not in the Neotropical region ), and Sprentascaris whose members are restricted to South America and were only reported in freshwater fishes ( Ramallo 2009). Species of Raphidascaris (Sprentascaris) are characterized by possessing a cuticular ring without spines, the presence of small postlabial or interlabial cuticular elevations, absence of true interlabia; the excretory pore slightly posterior to nerve ring level, a muscular oesophagus with ventriculus and ventricular appendix; and no intestinal caecum ( Moravec 1998).
All species of Raphidascaris (Sprentascaris) , except R. (S.) lanfrediae , have Siluriformes fishes as hosts. Raphidascaris (S.) hypostomi was isolated from specimens of the subfamilies: Hypostominae and Ancistrinae (all Loricariidae ) ( Moravec 1998). Eiras et al. (2010) found nematodes identified as R. (S.) hypostomi and R. (S.) mahnerti in Metynnis lippincottianus Cope ( Characiformes , Serrasalmidae ) from Brazil. Raphidascaris (S.) mahnerti was also reported in Loricariidae fishes; moreover Moravec et al. (1993) recorded the third larval stage of Raphidascaris (Sprentascaris) in the intestine of the cichlid Geophagus brasiliensis Quoy & Gaimard , ( Perciformes , Cichlidae ). The larva is probably conspecific with R. (S.) mahnerti and G. brasiliensis could be a paratenic host ( Moravec 1998). This is not the only time Raphidascaris (Sprentascaris) was detected in cichlids; Melo et al. (2011) reported the adults of R. (S.) lanfrediae in Satanoperca jurupari . Possibly, this species share the host spectrum with R. (S.) mahnerti . Both species might be closely related but they can be differentiated by morphological features and their geographical distribution (Melo et al. 2011). Raphidascaris (S.) pimelodi is distinguished from other known species of the subgenus, because it was only detected in a pimelodid species.
Although R. (S.) marano and Raphidascaris (S.) saltaensis sp. nov. have both been recorded in Northwest of Argentina, they inhabit different fluvial systems; R. (S.) marano in Marapa River (basin of Sali River, provinces of Tucumán and Catamarca), and R. (S.) saltaensis sp. nov. in La Caldera River (basin of the Bermejo River , province of Salta) . Ramallo (2009) proposed that R. (S.) marano would be an endemic species in Argentina, following the endemic character of its host, H. cordovae (Loricariidae) View in CoL . However, H. cordovae View in CoL is synonymous of H. paranensis , thus the geographical distribution was extended to include Paraguay (Paraguay River basin, the main tributary of Paraná River) ( Ferraris 2007). The new parasite species was found from Rineloricaria steinbachi (Loricariidae) , whose geographical distribution is limited to southern Bolivia and northwestern Argentina , including the basins of the Bermejo (Rivers Bermejo, San Andrés, La Caldera, Mojotoro and Vaqueros and streams Gallinato and Pucheta), Juramento (Rivers Salado, Piedras, Arias Arenales, Rosario, Aguas Negras and Calchaquí), Pilcomayo River , and also the Dorado River basin (La Sala stream and Popayan River ).
In this paper, we propose the erection of the sixth species of Raphidascaris (Sprentascaris) from the Neotropical region and the second from Argentina. Raphidascaris (S.) saltaensis sp. nov. differs from all congeners in the number of preanal papillae, the shape and size of lips, the egg’s shell and the spicules length. The new identification key presented in the article incorporates R. (S.) lanfrediae , R. (S.) marano and R. (S.) saltaensis sp. nov.
TABLE 1. Measurements of Raphidascaris (Sprentascaris) saltaensis sp. nov. Given in mm, mean ± SD (range). (* measurements with a unique value).
| Character | Holotype | Paratypes | |
|---|---|---|---|
| ♀ 1 | ♀ 5 | ♂ 8 | |
| Total body length | 6.86 | 6.59±1.37 (5.62–7.56) | 5.65±0.32 (5.12–5.92) |
| Body width | 0.57 | 0.58±0.06 (0.54–0.63) | 0.46±0.15 (0.28–0.67) |
| Lateral alae width | 0.01 | 0.02* | 0.02±0.02 (0.01–0.04) |
| Lips length | 0.06 | 0.05±0.02 (0.04–0.06) | 0.06±0.00 (0.06–0.07) |
| Oesophagus total length | 0.75 | 0.92±0.25 (0.71–1.27) | 0.79±0.09 (0.63–0.88) |
| Oesophagus total width | 0.30 | 0.36±0.06 (0.30–0.43) | 0.22±0.06 (0.14–0.34) |
| Nerve ring—anterior end | 0.29 | 0.37±0.08 (0.32–0.43) | 0.34±0.03 (0.29–0.37) |
| Excretory pore—anterior end | 0.36 | 0.45±0.02 (0.44–0.47) | 0.40±0.05 (0.32–0.47) |
| Ventriculus length | 0.05 | 0.07* | 0.06±0.01 (0.06–0.07) |
| Ventriculus width | 0.13 | 0.13* | 0.19±0.06 (0.15–0.24) |
| Ventricular appendix length | 0.16 | 0.10* | 0.14±0.06 (0.10–0.19) |
| Ventricular appendix width | 0.06 | 0.04* | 0.05±0.00 (0.04–0.05) |
| Ventricular appendix/ oesophagus length ratio | 1: 0.21 | 1:0.13* | 1:0.21 (1:0.12–0.3) |
| Vulva—anterior end | 1.20 | 1.74* | - |
| Spicules length | - | - | 0.24±0.02 (0.22–0.27) |
| Tail length | 0.37 | 0.30±0.13 (0.15–0.39) | 0.25±0.02 (0.22–0.27) |
| Eggs length | 0.07 | 0.06±0.02 (0.04–0.09) | - |
| Eggs width | 0.04 | 0.03±0.01 (0.03–0.04) | - |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |