Malacomorpha cyllarus ( Westwood, 1859 )
|
publication ID |
https://doi.org/10.11646/zootaxa.1748.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD87F7-9E74-FFEC-C3C3-FC01FE6AFE27 |
|
treatment provided by |
Felipe |
|
scientific name |
Malacomorpha cyllarus ( Westwood, 1859 ) |
| status |
|
Malacomorpha cyllarus ( Westwood, 1859) View in CoL
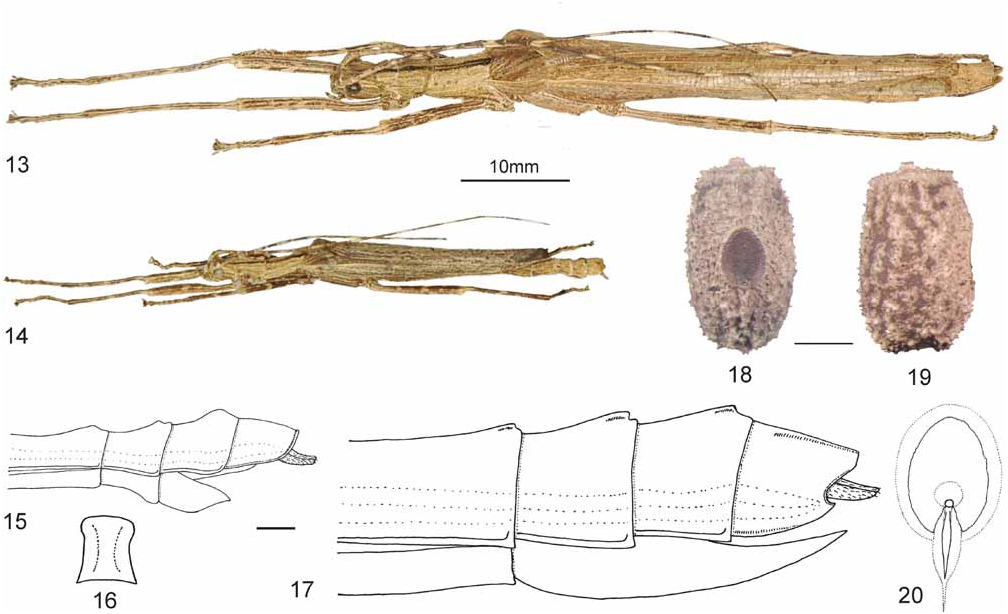
( Figs. 13–20 View FIGURES 13–20 , 83–84 View FIGURES 82–85. 82 )
Necroscia cyllarus Westwood, 1859: 155 , pl.13: 2 ( ♂), pl.14: 5 ( ♀). LT, ♂: 397, ♂, Jamaica; cyllarus Westw. , Necroscia cyllarus Westwood, ST , Necroscia cyllarus Westw. (BMNH) ; PLT, ♀: Jamaica, 402, 397; ♀, cyllarus Westw. , Pseudophasma View in CoL , Necroscia cyllarus Westw. , Necroscia cyllarus Westwood, ST (BMNH) . [examined]
Redtenbacher, 1906: 127. [As “Species incertae sedis”]
Pseudophasma cyllarus, Kirby, 1904: 411 View in CoL .
Rehn, 1904: 99.
Anisomorpha cyllarus, Langlois & Lelong, 1996: 22 View in CoL .
Malacomorpha cyllarus, Conle & Hennemann, 2002: 49 View in CoL , pl. 6: 54–55 ( ♂, ♀), pl. 12: 117–118 (genitalia), pl. 16: 177–178 (egg), 19: 208. [Designation of lectotype]
Zompro, 2004: 147, fig. 9: 4 (egg).
Otte & Brock, 2005: 392.
Phasma graveolens King, 1867: 78 . Type (s): Jamaica, Santa Cruz Mountains (depository unknown—presumed lost). n. syn. [Not: Phasma graveolens, Caudell, 1909: 111 . Misidentification, = Anisomorpha buprestoides Stoll, (1813) View in CoL ]
Material examined [ 28 ♂♂, 12 ♀♀, 3 ♀♀ nymphs]: 1 ♂, 1 ♀: Jamaica, F. Klages, Coll. W. J. Holland, ANSP, Ex Carn. Mus. Bruner Cln. ( ANSP) ; 1 ♀ (nymph): Malvern , Jamaica, Petrunkevitch, MCZ ( ANSP) ; 2 ♂♂: Montego Bay , Jamaica, Mar. 14, 1911, Anisomorpha cyllarus (Westw.) det. Hebard 1924 ( ANSP) ; 1 ♂, 1 ♀: Jamaica, St. Ann Parish Rose Hill , 95m, Runaway Bay, 1. May 1973, Don & Mignon Davis ( USNM) ; 1 ♂: Jamaica, St. Ann Par. Discovery Bay , 27.–28. February 1984, J.M. Carpenter ( USNM) ; 1 ♂: Snug Harbor , Montego Bay, Jamaica, 6.26.10, E.A. Andrews Coll., USNM, ANSP, Anisomorpha cyllarus (Westw.) det. C.F. Moxey 1972 ( USNM) ; 1 ♂, 3 ♀♀: 92–32, Jamaica ( BMNH) ; 3 ♂♂: ex Zucht O. Conle 2001 ( MNHU) ; 2 ♂♂, 1 ♀: ex Zucht 2001 O. Zompro, Zuchtstamm Jamaika ( OC) ; 16 ♂♂, 4 ♀♀, 2 ♀♀ (nymphs): ex Zucht O. Conle 2002, Zuchtstamm aus Jamaika ( OC) ; 1 ♂, 2 ♀♀, eggs: ex Zucht : F. Hennemann, urspr. Jamaika, 2001–2002 (FH, No’s 0490-1 to 3 & E) .
Distribution: Jamaica (Montego Bay: St. James [ Moxey, 1972: 30] & Snug Harbour; Runaway Bay; Discovery Bay: St. Ann Parish Rose Hill 95m; Kingston [ Rehn, 1904: 99]; Long Mountain [ Moxey, 1972: 30], St. Andrew [ Moxey, 1972: 30]; Clarendon: Portland Ridge [ Moxey, 1972: 30]; Trelawny: Falmouth [ Moxey, 1972: 30]; Santa Cruz Mountains: Belmont [ King, 1867: 78] & Malvern
Differentiation: Similar to the species of the genus with fully developed alae in both sexes: Malacomorpha spinicollis ( Burmeister, 1838) & Malacomorpha hispaniola n. sp.; and in the male similar to Malacomorpha poeyi ( Saussure, 1868) .
From Malacomorpha spinicollis ( Burmeister, 1838) it differs by: the smaller size; more slender body and legs; lack of spines on the mesonotum, paler and less speckled coloration of the body and legs of both sexes. From Malacomorpha hispaniola n. sp. it differs by: the mesonotum being longer in relation to the pronotum, the shorter alae which only reach to the posterior margin of of tergite VII or VIII of both sexes; the vomer without serration of ♂♂.
From the ♂ of M. poeyi ( Saussure, 1868) it differs by: the smaller size and the shorter and more robust body.
The eggs differ from all other known eggs of the genus by the very prominently sculptured, scabrous capsule surface which is covered all over with tooth-like structures.
Description: The colouration is described from live specimens.
ºº ( Figs. 13 View FIGURES 13–20 & 83 View FIGURES 82–85. 82 ): Large (body length 57.0–65.0 mm), slender for the genus with a rather cylindrical abdomen. Tegmina and alae present. Alae reaching towards the posterior end of tergite VII or even VIII. Legs slender but not very long, distinctly carinated; all carinae covered with minute setae. Antennae long and slen- der, reaching to posterior margin of anal segment. Body surface minutely tuberculose and rugulose, not shiny except dorsal surface of abdomen; mesonotum bearing several minute tubercles roughly arranged in four longitudinal rows in the anterior half. Basic colouration of body and legs pale brown, overlaid with many minute dark brown speckles and broken lines. A prominent, dark longitudinal dorsomedian line runs along the complete dorsal surface of the head and thorax, becoming more indistinct or even absent towards the end of the abdomen. Tegmina and costal region of alae pale brown sometimes with indistinct brown mottles. Anal region of alae translucent. Head with several indistinct, pale and dark brown longitudinal dorsolateral lines and sometimes with an indistinct dark brown postocular line. Antennae pale to mid brown with irregular yellowish bands, the antennomeres irregularly coloured. Eyes marbled in black and mid brown. Legs pale brown with indistinct yellowish and dark brown mottling and minute spots.
Head: Slightly longer than wide, oval in cross-section and slightly flattened dorsally. Vertex smooth. Rudiments of ocelli present. Eyes large, roughly circular, distinctly projecting hemispherical, their length contained 1.5x in that of cheek. Antennae reaching to posterior margin of anal segment. Scapus 1.5x longer than wide, compressed dorsoventrally, roughly rectangular and slightly carinated. Pedicellus as long as wide, distinctly narrower and about half as long as scapus, but wider than following antennomeres. Third antennomere elongate, almost as long as scapus and pedicellus combined, IV distinctly shorter. Remaining antennomeres increasing in length towards apices of antennae.
Thorax: Oval in cross-section. Pro- and metathorax parallel-sided, only mesothorax distinctly broadened towards the posterior. Pronotum as wide as but 1.5x longer than head, 1.5x longer than wide, parallel-sided. Anterolateral angles with a conspicuous, rounded excavation for the defensive glands. Transverse median depression indistinct and slightly displaced towards anterior third of segment. Median line slightly impressed. Mesonotum hardly wider and almost 1.5x longer than pronotum, 2x longer than wide and parallel-sided. Bearing several minute tubercles roughly arranged in two dorsolateral and two lateral longitudinal rows in the anterior half. Metanotum and median segment wider than mesonotum and combined longer than mesonotum. Metanotum and median segment combined hardly 2x longer than wide, parallel-sided, smooth and shiny, covered by the tegmina and alae. Metanotum transverse, wider than long and slightly shorter than median segment. Slightly impressed median line continued from the mesonotum. Transverse fissure between metanotum and median segment very distinct and almost straight. Meso-, metaepisternum and pro- and metasternum simple and smooth. Mesosternum with moderate longitudinal ventromedian carina. Tegmina short and oval, strongly convex, bearing fine veins, reaching towards the posterior margin of metanotum. Alae reaching towards the posterior end of tergite VII or even VIII.
Abdomen: 1.5x longer than head and complete thorax combined, slender and gently gradually tapered towards the apex. Surface smooth, dorsal area covered by the closed alae, shiny. Median segment slightly longer than metanotum, gently wider than long, rectangular with transverse impressed fissure in the centre. Tergites parallel-sided. II–VI widest and longest, VIII–X narrowest and shortest. II–VII roughly quadrate, VIII & IX transverse, 1.5–2.0x wider than long. Tergites VII–IX each with a minute faint posteromedian tubercle or hump (sometimes almost absent). Sternites II–VI simple and smooth, VII bearing a small black praeopercular organ. Anal segment parallel-sided towards apex, posterior margin laterally slightly broadened, narrower than IX, about 1.5x wider than long, with an indistinct longitudinal median carina. Lateral margins with a faint concave excavation near the bases of the cerci. Supraanal plate very small with angulate apex just visible. Subgenital plate boat-shaped, with faint ventromedian longitudinal impression; reaching the posterior marging of anal segment; minutely setose and apex pointed. Cerci small, short, slightly incurving, and gradually constricted towards the apex, which is slightly thickened and club-like; finely bristled.
Legs: Rather slender and not very long, distinctly carinated, unarmed and with all carinae minutely bristled. Profemora at least 2x longer than mesothorax, metafemora reaching to posterior margin of abdominal tergite IV, hind legs hardly projecting over apex of abdomen. Profemora considerably compressed and curved basally. Basitarsus 2x longer than second tarsomere.
ďď ( Figs. 14 View FIGURES 13–20 & 84 View FIGURES 82–85. 82 ): Similar to ♀♀, but smaller and much more slender (body length 33.0–40.0 mm), abdominal segments II–VII parallel-sided.
Head: Generally as in ♀♀.
Thorax: As in ♀♀, but mesothorax less distinctly broadened towards the posterior.
Abdomen: Sub-cylindrical in cross section, about 1.5x longer than head and thorax combined. Surface and granulation as in ♀♀. Tergites II–VII parallel-sided, VIII and IX broadening towards the posterior and broader than previous. III–VII are the longest and narrowest, IX is the shortest, X is the widest. II–VII 1.5 – 2x longer than wide, VIII & IX 1.5–2.0x wider than long, anal segment broader than previous tergites, about 2x wider than long. Posterior margin rounded, swollen and laterally expanded, with a very small median indentation. Sternites II–VII simple and smooth. Cerci as in ♀♀ but slightly longer. Poculum small and flat, spoon-like, reaching towards the posterior margin of tergite IX. Posterior margin rounded with minute pointed apex medially. Vomer longer than wide, parallel-sided basally, with apex broadly rounded; outer margin swollen.
Legs: As in ♀♀.
Eggs ( Figs. 18–20 View FIGURES 13–20 ): As in other species of the genus the eggs present considerable variation concerning to the size, colour and sculpturing of the capsule. Average eggs were used for the descriptions provided below.
Of moderate size for the genus. Capsule barrel-shaped, 1.6–1.8x longer than wide, oval in cross-section, lateral surfaces gently convex. Polar-area flattened and with a distinct impression if seen in lateral aspect. Anterior margin of capsule raised and strongly armed with rugulose or tooth-like structure. Entire surface of capsule strongly scabrous, rugulose and all over covered with irregular spine or tooth-like structures, which become conspicuously more decided and numerous towards the dorsal egg surface and micropylar plate. Structures forming two shallow, irregularly raised sub-parallel longitudinal carinae beginning at the anterior end of the micropylar plate and ending at anterior margin of capsule. Two further more distinct but irregular, slightly converging ridges reach from the posterior end of the micropylar plate almost to the polar area. Micropylar plate rather large, oval, 1.2–1.4x longer than wide and slightly less than 1/3 the length of capsule. Surface gently concave and very minutely granulose. Micropylar cup very small and positioned close to the posterior margin of micropylar plate; oval. Median line indistinct, very fine and almost reaching to polar area; laterally accompanied by an irregular ridge. Operculum oval, very slightly convex and with a prominent, conical to knob-like hump in the centre; otherwise structured like capsule. General colouration varying from plain pale greenish over pale to mid brown, impressed portions in between the raised structures darker brown. Micropylar plate plain dark brown.
Measurements [mm]: length 2.8–3.6, width 1.7–2.1, height 1.9–2.3, length of micropylar plate 0.9–1.0.
Comments: The systematic position of this species has been a problem since it was originally described. Westwood (1859: 155) described Necroscia cyllarus from a ♂♂ and ♀♀ from Jamaica in BMNH and provided very accurate illustrations of both sexes (pls. 13: 2 & 14: 5). Kirby (1904: 411) transferred it to Pseudophasma Kirby, 1896 and Rehn (1904: 99) recorded a ♂♂ from Kingston ( Jamaica) in USNM and already quoted a rather aberrant position of N. cyllarus in the genus Pseudophasma Kirby. Redtenbacher (1906: 127) listed it as „species incertae sedis“ in his tribe Phasmini , section Phasmata. Finally, Conle & Hennemann (2002: 49) recognized its close relation to Malacomorpha androsensis Rehn, 1906 and consequently placed N. cyllarus in Malacomorpha Rehn, 1906 . These authors furthermore designated a lectotype and provided descriptions and illustrations of both sexes and the eggs of M. cyllarus . Zompro (2004: 148) surprisingly doubted the generic placement of M. cyllarus and stated that the eggs “differ considerably” without having seen eggs of the type species.
Although Phasma graveolens King, 1867 was described from Jamaica Caudell (1909: 111) synonymised this species with the continental American Anisomorpha buprestoides (Stoll, 1813) . This is obviously not correct, since King (1867 78) described his graveolens to have well developed alae, which is not true for any member of Anisomorpha Gray. The original description by King and type-locality (Belmont in the Santa Cruz Mountains of Jamaica) do instead match very well with M. cyllarus and show King’s species to be a synonym of M. cyllarus ( Westwood, 1859) ( n. syn.).
Along with the description of Phasma graveolens King (1867: 79) provided some interesting information on the native food-plant, biology and behaviour of M. cyllarus in Jamaica. King stated it to be apparently numerous near Belmont (Santa Cruz Mountains) from May to July and that several hundred adult couples are frequently found at night almost exclusively on shrubs of Bignonia chinensis (Bignoniaceae) , which is the preferred food in that locality. During the day King (1867: 79) reported the nymphs and adults to hide in the holes of trees, amongst brushwood where it is sufficiently dense to exclude the light, and also in the cellars and behind the boarding of houses.
This species is frequently reared in captivity in Europe since the late 1990’s from a stock collected in central Jamaica by Pat & Tony James ( England). As alternative food-plants various sorts of privet ( Ligustrum spp. , Oleaceae ) are readily accepted by European cultures. It is contained on the “Phasmid Study Group” culture-list as culture No. 220.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Malacomorpha cyllarus ( Westwood, 1859 )
| Conle, Oskar V., Hennemann, Frank H. & Perez-Gelabert, Daniel E. 2008 |
Malacomorpha cyllarus
| Conle, O. V. & Hennemann, F. H. 2002: 49 |
Anisomorpha cyllarus
| Langlois, F. & Lelong, P. 1996: 22 |
Pseudophasma cyllarus
| Kirby, W. F. 1904: 411 |
Phasma graveolens
| Caudell, A. N. 1909: 111 |
| King, C. B. 1867: 78 |
Necroscia cyllarus Westwood, 1859: 155
| Westwood, J. O. 1859: 155 |