Nemateleotris lavandula, Tea & Larson, 2023
|
publication ID |
https://doi.org/ 10.26107/RBZ-2023-0019 |
|
publication LSID |
lsid:zoobank.org:pub:1D807595-BF4C-4B0D-A87F-65EF50950A8B |
|
persistent identifier |
https://treatment.plazi.org/id/74B57DF1-1966-404F-9CE9-562957D96357 |
|
taxon LSID |
lsid:zoobank.org:act:74B57DF1-1966-404F-9CE9-562957D96357 |
|
treatment provided by |
Felipe |
|
scientific name |
Nemateleotris lavandula |
| status |
sp. nov. |
Nemateleotris lavandula , new species
Lavender-blushed Dartfish ( Figs. 2H, 4D–F View Fig , 5 View Fig , 6 View Fig , 7 View Fig , 8B, C View Fig , 9B View Fig , 10E View Fig 1– E View Fig 2, 11; Tables 1, 2)
Nemateleotris helfrichi View in CoL (in part, non Randall & Allen, 1973): Randall & Allen, 1973: 356, fig. 5 (part of description of N. helfrichi View in CoL ; underwater photograph from Palau); Myers, 1988 (checklist; Marianas Islands); Myers, 1989: pl. 118D (field guide, aquarium photograph of specimen from Micronesia); Rivaton et al., 1990: 41 (distribution record from New Caledonia); Myers, 1999: pl. 164G (field guide, underwater photograph from Micronesia); Rivaton & Bourret, 1999: 172 (checklist; New Caledonia); Laboute & Grandperrin, 2002: 420 (field guide, underwater photograph from New Caledonia); Larson, 2001: 3608 (identification guide to species of Microdesmidae View in CoL ); Allen et al., 2003: 282 (field guide, underwater photograph); Randall, 2005: 564 (field guide, underwater photograph from Marshall Islands); Kuiter & Debelius, 2006: 666 (underwater photograph from Micronesia); Fricke et al., 2011b (checklist; Vanuatu); Fricke et al., 2011a (checklist; New Caledonia); Allen & Erdmann, 2012: vol. 3, 994 (underwater photograph from Fiji); Randall & Connell, 2013: fig. 4 (underwater photograph from Kwajalein Atoll, Marshall Islands); Allen et al., 2015: 286 (field guide, underwater photograph); Koeda et al., 2016: fig. 452 (checklist; underwater photograph from Yonaguni Island, Japan); Motomura & Harazaki, 2017: pl. 10G (checklist; Yakushima Island, southern Japan); Coleman et al., 2018 (checklist; Pohnpei, Federated States of Micronesia); Nakae et al., 2018 (checklist; Amami-oshima Island, Ryukyu Islands, Japan); Rosenstein, 2019: 272 (field guide; underwater photograph from Fiji).
Holotype. AMS I.16876-001 (also paratype of N. helfrichi ), 45.6 mm SL, Augulupelu Reef , Palau Islands, 28 m, multiprong spear, W.A. Starck II, 4 March 1972.
Paratypes (n=12). AMS I.18411-017 (previously AMS I.18411-010), 46.9 mm SL, Suva Harbour , Fiji, 30–37 m, B. Carlson, B. Goldman, & P. Colin, 16 February 1974 ; BPBM 9527 (also paratype of N. helfrichi ), 25.0 mm SL, Cocos Island , Guam, Mariana Islands, 27.5 m, rotenone, J.E. Randall, P. Helfrich, R.S. Jones, & H. Kami, 28 May 1968 ; BPBM 10153 (also paratype of N. helfrichi ), 30.9 mm SL, outer reef slope of Rigili Islet , Eniwetok Atoll, Marshall Islands, 34 m, quinaldine, G.R. Allen, 30 August 1970 ( Fig. 4D View Fig ) ; BPBM 12661 (also paratype of N. helfrichi ), 2, 33.5–35.5 mm SL, outer reef slope of Rigili Islet , Eniwetok Atoll, Marshall Islands, 43 m, rotenone, J.E. Randall, 7 April 1972 ; BPBM 19974 , 45.4 mm SL, outer reef slope of Kwajalein Atoll, Marshall Islands, 50 m, rotenone, J.E. Randall, N.A. Bartlett, R. Hergenrother, & K. Burnett, 8 April 1976 ; BPBM 40810 , 38.5 mm SL, Alet Islet , Puluwat Atoll, Caroline Islands, 10–25 m, quinaldine, R.L. Pyle & B.D. Greene, 11 April 2007 ; CAS-ICH 27591 (also paratype of N. helfrichi ), 35.5 mm SL, Augulupelu Reef , Palau Islands, 28–61 m, rotenone, G.R. Allen & W.A. Starck II, 1 March 1972 ; KPM-NI 10136 View Materials , 39.0 mm SL, Ototo-jima , Chichijima, Ogasawara Islands, Japan, 45 m, dip net, O. Morishita & Ehara, 28 August 1995 ; USNM 209306 (also paratype of N. helfrichi ), 51.5 mm SL, Augulupelu Reef , Palau Islands, 37 m, multi-prong spear, W.A. Starck II, 10 January 1972 ; ZRC 62990 , 2 , 29.8–36.1 mm SL, aquarium specimens collected from Kwajalein Atoll, Marshall Islands ( Fig. 4E–F View Fig ) .
Diagnosis. Nemateleotris lavandula is most similar to N. helfrichi , sharing with it the following combination of characters and live colouration details to the exclusion of all other Nemateleotris : caudal fin truncate to weakly emarginate; dorsoposterior ctenoid scales with fewer than 10 ctenii; elevated portion of first dorsal fin blue on anterior edge; median fins pale yellowish green, caudal fin without any markings, outermost edge of second dorsal and anal fin tipped with a yellow or orange spot, one in each interradial membrane space; body lavender to lilac in life; pelvic fins black-tipped; dorsal edge of iris with a black mark at 1 o’clock position, sometimes continuing onto interorbital space as a short streak. It is readily separated from N. helfrichi and all other congeners based on the following: maxilla unmarked (bright yellow in life, pale tan in preservation); and snout, lower jaw, preopercle, and postorbital region bright yellow in life.
Description. Dorsal-fin rays VI–I,30 (I,29–30), all segmented rays unbranched; anal-fin rays I,27 (I,26–I,28), all segmented rays unbranched; pectoral-fin rays 20/19 (18–20), upper and lowermost 3–4 unbranched, all other rays branched (all pectoral-fin rays unbranched in BPBM 10153; BPBM 12661 [33.5 mm SL specimen]; and ZRC 62290 [29.8 mm SL specimen]); pelvic-fin rays I,5; segmented caudal-fin rays 17; upper procurrent caudal-fin rays 10 (10–14); lower procurrent caudal-fin rays 10 (10–14); total caudal-fin rays 37–45; no visible lateral line; longitudinal scale series 125 (125–140); gill rakers 6 + 17 (17–18) = 23 (23–24). Body elongate and compressed, depth 5.8 (5.0–6.2) in SL, width 1.6 (1.6–2.9) in depth; head 4.5 (3.8–5.1) in SL; snout 4.6 (4.6–7.8) in head; orbit diameter 2.4 (2.3–3.1) in head; bony interorbital space flat, least width 3.9 (3.5–5.1) in head; caudal peduncle short, deeper than long, least depth 1.8 (1.8–2.5) in head.
Mouth strongly oblique, forming at angle of about 50° to horizontal axis of body; maxilla reaching a vertical through centre of eye, upper jaw 3.4 (2.7–4.3) in head; upper jaw with an outer row of four to six, widely spaced, moderately large, incurved canines on each side, and a medial band of small villiform teeth that narrows posteriorly; lower jaw slightly protruding when mouth closed, with three enlarged recurved canines at corner of each side of jaw, more posterior tooth largest, a middle band composed of two to four rows of low villiform teeth narrowing to a single row posteriorly, and an inner pair of enlarged recurved canines at front corner of each side; no teeth on vomer or palatines; tongue truncate, set far back in mouth.
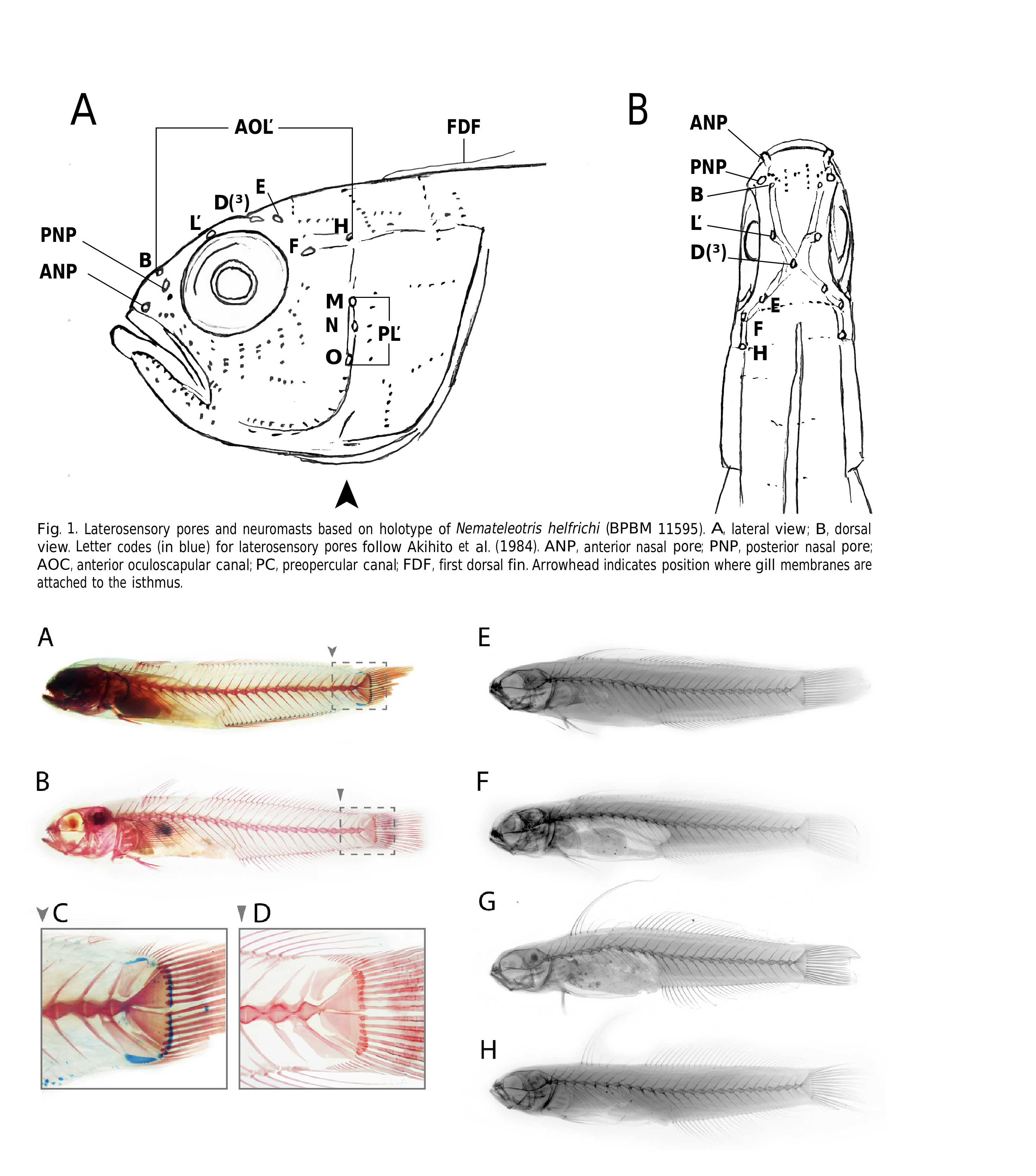
A low median fleshy ridge on top of head from interorbital space to origin of first dorsal fin; no opercular or preopercular spines; preopercular margin free only ventrally; upper end of gill opening at or slightly dorsal to level of middle of eye, the ventral end extending to below posterior margin of preopercle; anterior and posterior nasal pore separated by a distance about equal to half the pupil diameter; anterior nasal pore small, rounded and terminating in a short fleshy tube; posterior nasal pore larger, rounded, with little or no rim.
No visible lateral line on body; pores of cephalic lateral line system as described above in generic diagnosis; scales small, ctenoid posteriorly on body to about posterior third of dorsal fin, cycloid elsewhere, those anteriorly frequently embedded; no scales on head; no median predorsal scales, but embedded scales extend anteriorly on side nape to level of gill opening or slightly beyond; prepelvic area of thorax with embedded scales; anterior portion of isthmus naked; no scales on fins except for approximately basal half of caudal fin.
First dorsal fin elevated anteriorly, first spine longest, 3.6 (3.0–3.9) in SL, second and third spines only slightly shorter; fifth spine 2.0 (2.0–3.5) in head; sixth spine 3.2 (2.6–3.9) in head; spine of second dorsal fin 3.6 (2.7–4.0) in head;
RAFFLES BULLETIN OF ZOOLOGY 2023
penultimate dorsal-fin ray usually longest, 1.3 (1.3–2.7) in head; caudal fin truncate or weakly emarginate, lobe tips rounded, fin length 4.5 (4.3–5.1) in SL; pectoral fins moderately pointed, middle rays longest, 4.7 (4.5–6.1) in SL; pelvic fins separate, their origin directly below pectoral-fin base, length 6.3 (5.1–7.4) in SL.
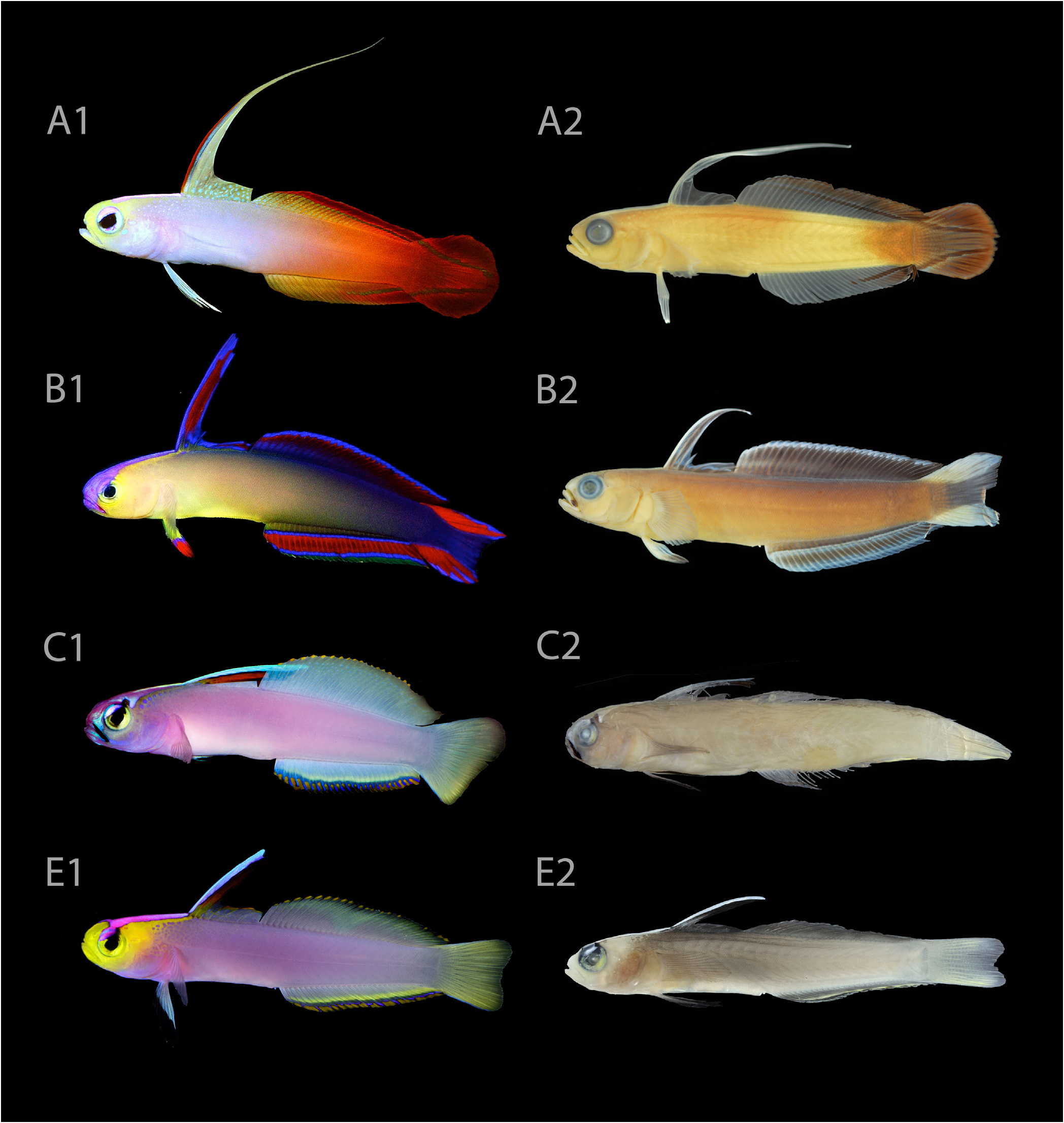
Colour in life. Based on colour photographs of specimens when freshly dead, and live individuals in the field and aquaria ( Figs. 4D–F View Fig , 6 View Fig , 7 View Fig , 8B, C View Fig , 9B View Fig , 10 E View Fig 1 View Fig , 11): snout, jaws, cheeks, preopercle, and postorbital region bright yellow, remainder of head lavender to lilac; body lavender to lilac, becoming increasingly pale posteriorly toward caudal peduncle; postorbital yellow marking continuing to anterior first dorsal fin as a sharp streak, becoming increasingly anastomosed and suffused away from the head; iris bright yellow, dorsal edge sharply capped in metallic purple with a black mark at 1 o’clock position, sometimes continuing onto interorbital space as a short streak; interorbital region with bright metallic pink triangle, its base originating from middle of interorbital space, narrowing along entire length of predorsal space before terminating at origin of first dorsal fin; first two interspinous membrane spaces on elevated portion of first dorsal fin blue; adjacent interspinous membrane space between 2 nd and 3 rd first dorsal-fin spines red, sometimes dusky red; remaining portion of first dorsal fin translucent lavender, often edged in yellow or orange; second dorsal fin pale yellowish green, distalmost edge with a series of bright yellow or orange spots, one in each interradial membrane space, spots sometimes coalescing; anal fin similar to second dorsal fin, except edged submarginally in bright yellow and bright blue; distalmost edge of anal fin with a series of bright yellow or orange spots, one in each interradial membrane space, spots sometimes coalescing; caudal fin pale yellowish green, slightly translucent on outer edge; pelvic fins pale blue to white, black tipped distally; pectoral fins transparent.
Colour in preservation. ( Fig. 10E View Fig 2): similar to colour in life, except body now purplish grey (fading to tan over time); head and snout now pale tan; interorbital and predorsal triangle of colour now pale grey, bordered in black; first two interspinous membrane spaces on elevated portion of first dorsal fin now white to pale grey; all black markings remain; median fins translucent hyaline.
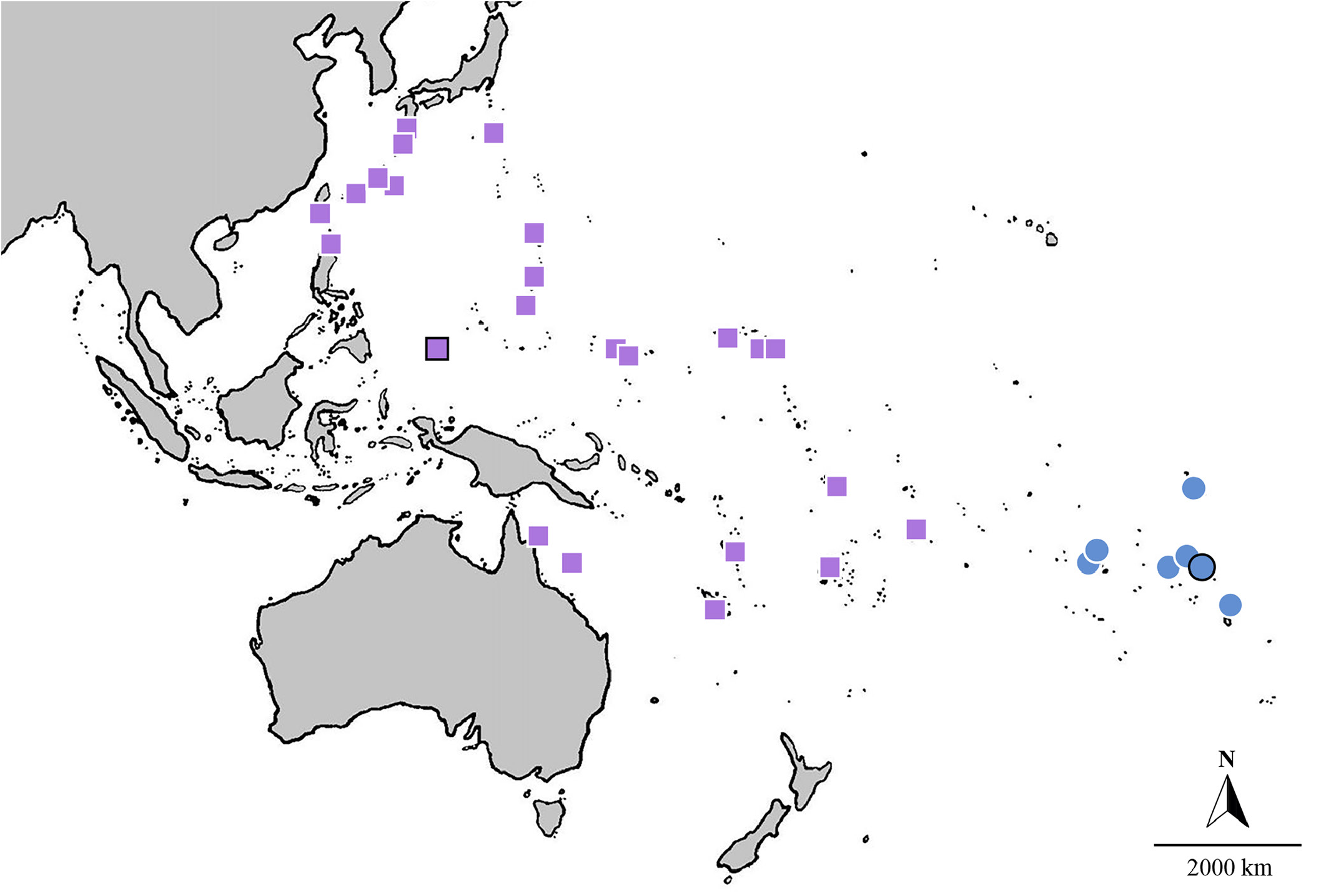
Habitat and distribution. Nemateleotris lavandula is widespread across much of the western and central Pacific Ocean ( Fig. 5 View Fig ). Its distribution follows the northwestern contours of the Pacific Plate , from Yakushima Island in southern Japan, throughout the Ryukyu and the Ogasawara Islands , and south to Taiwan and the northern Philippines, extending east across Micronesia, including the Caroline Islands, the Marshall Islands, and the Mariana Islands. In Melanesia , the species has been reported from Fiji, Vanuatu, and New Caledonia. It has also been reported from Samoa, American Samoa, Tonga, the Coral Sea, and Hicks Reef on the Great Barrier Reef. It frequents seaward sand channels and rubble pans adjacent to coral reefs at depths between 25–100 m.
Etymology. The species is named lavandula , after the genus of flowering plants which includes the ornamental herb lavender, in reference to its beautiful colouration in life. To be treated as a noun in apposition.
Comparisons. Nemateleotris lavandula most closely resembles N. helfrichi , sharing similarities in meristics, morphometrics, and live colouration. Molecular analysis of mitochondrial COI reveals a difference of 1% between both species (uncorrected pairwise distance). This value is lower than the usual threshold of 2–6% between congeneric sister species of coral reef fishes, but higher than most intraspecific variation within species ( Steinke et al., 2009). In any case, it is not uncommon for many groups of coral reef fishes to exhibit little or no mitochondrial differentiation, particularly in recently diverged groups where incomplete lineage sorting has occurred, or between groups that have undergone recent introgression. Both species are united in having the following combination of live colouration details not found in other species of Nemateleotris : elevated portion of first dorsal fin blue on anterior edge; median fins pale yellowish green, caudal fin without any markings, outermost edge of second dorsal and anal fin tipped with a yellow or orange spot, one in each interradial membrane space, spots sometimes coalescing; body lavender to lilac in life; pelvic fins black-tipped; and dorsal edge of iris with a black mark at 1 o’clock position, sometimes continuing onto interorbital space as a short streak.
Nemateleotris lavandula differs from N. helfrichi primarily in lacking a black mark on its maxilla (in life and preservation; Fig. 8C View Fig ) and in having a bright yellow head and snout (vs pink snout and head only weakly suffused with yellow in N. helfrichi ; Fig. 9A–B View Fig ). It further differs from N. helfrichi in having a wider interorbital width (5.1–6.0% SL vs 2.2–3.4% SL) and a larger orbit (8.2–9.4% SL vs 6.6–7.9% SL). It differs from N. decora in having fewer anal-fin rays (26–28 vs 28–32) and in having the posterior most dorsal- and analfin rays usually unbranched (versus usually branched in N. decora ), and from N. magnifica in having a shorter first dorsal-fin spine (more than 2.5 in SL and non-filamentous vs up to 1.4 in SL and filamentous), a different caudal fin shape (truncate to weakly emarginate vs round), and in having smaller ctenoid scales on the dorsoposterior body with fewer ctenii (10 or less vs more than 15). Differences in live colouration for all species of Nemateleotris are summarised in Figs. 8 View Fig , 9 View Fig , 10 View Fig , and Table 2.
Status of Nemateleotris exquisita . Randall & Connell (2013) described N. exquisita on the basis of 12 specimens (erroneously listed as nine and eleven specimens on separate occasions in the same publication) collected from various localities in the Indian Ocean, including Mauritius (type locality), South Africa, and the Red Sea. They distinguished their new species from N. decora in having: a more slender body (body depth 5.7–6.3 in SL vs 4.9–5.7 in SL); a longer snout (4.0– 4.7 in head vs 5.0– 7.5 in head); an apparently smaller eye (proportional measurements not given); an apparently shorter first-dorsal fin spine (proportional measurements not given); and a greater maximum size (66.0 mm SL vs 52.5 mm SL; the latter based on examination of 15 specimens of N. decora ). They also distinguish N. exquisita from N. decora in having the body more yellow anteriorly (yellow extending beyond the anal-fin origin vs body whitish pale grey anteriorly to not beyond anal-fin). Their comparative data for N. decora used in the description of N. exquisita were taken from the original description, based on eleven specimens in the type series.
We examined seven paratypes of N. exquisita from the western Indian Ocean, as well as an additional five specimens and one paratype of N. decora from the Pacific Ocean. Based on revised comparative data collected, we find overlapping morphometric values for the following characters used in distinguishing N. exquisita from N. decora : body depth (5.1–6.3 in SL vs 5.7–6.1 in SL); and snout length (3.7–5.4 in head vs 4.0– 7.5 in head). One of our specimens of N. decora (AMS I.17500-017) had a standard length of 57.4 mm. Additionally, we note that both N. decora and N. exquisita typically display a wide range of variation in their anterior body colouration, with N. decora sometimes appearing exquisita -like in having a yellower anterior body, (see Fig. 10B View Fig 1 View Fig ; KPM-NR 49173; Fricke et al., 2011b: fig. 463), and N. exquisita sometimes appearing decora -like with reduced yellow pigmentation (see Tea et al., 2020a: fig. 2O).
The exact distribution of N. exquisita and N. decora appears to be contentious. In the description of N. exquisita , the authors report its occurrence in the Indian Ocean, from South Africa, Mauritius, the Red Sea, and east to the Andaman Islands. However, they commented on the possibility of a putative specimen of N. exquisita × N. decora photographed in the Maldives (based on a paler intermediate anterior body colouration), stating: “… N. decora presently known westward only to Sulawesi and Bali, and N. exquisita east to the Thai coast of the Andaman Sea, one might expect hybridisation could occur at some intermediate sites such as Sumatra. Because of its deep-reef habitat and being difficult to collect, we do not know the definitive distribution of N. exquisita . It might range to Sri Lanka like such species as Chaetodon andamanensis and Halichoeres timorensis and stray to the Maldives.” The last two sentences appear to be erroneous, and presumably the authors meant the Pacific Ocean N. decora as potentially straying as far west as Sri Lanka and the Maldives (not the Indian Ocean N. exquisita ) where it may hybridise with N. exquisita ( N. decora has been reported and photographed in Bali, Sumatra, and Christmas Island, these presumably representing the westernmost limit of its Pacific distribution).
Lastly, the authors compared mitochondrial COI barcodes for N. exquisita and N. decora , finding no differences in sequence data. They alluded to the possibility of incomplete lineage sorting or introgression being a possible explanation for the lack of sequence variability. While this phenomenon is indeed common for various groups of coral reef fishes ( Victor & Randall, 2014; Tea et al., 2016; Victor, 2016; Tea et al., 2020b), the lack of at least one unequivocally robust line of evidence justifying the distinction of N. exquisita and N. decora is concerning. Until shown otherwise, we recognise N. exquisita as a synonym of N. decora .
Biogeography and phylogenetic relationships. The biogeography of Nemateleotris is rather unusual, comprising only four species, but with three that are widely distributed and one endemic to several island groups. Springer (1982) made note of this in his contributions to Pacific Plate biogeography, further adding that while it was not possible to determine the relationships among Nemateleotris , N. decora and N. helfrichi sensu lato (= N. helfrichi + N. lavandula ) showed greater morphological similarities to each other than either does to N. magnifica . While the evidence was slight, he proposed the possibility of an Indo-West Pacific species, in this case N. decora , being sister to the Pacific Plate endemic N. helfrichi s.l., with both species having undergone slight dispersal since their divergence leading to sympatry of both species.
Indeed, sympatry for at least three species of Nemateleotris , namely N. decora , N. lavandula , and N. magnifica , occurs in several parts of the western Pacific, particularly along the western margins of the Pacific Plate. Except for N. magnifica , which occurs from depths between 6–70 m (but not usually below 28 m), the remaining species of Nemateleotris primarily occur in mesophotic coral reefs at depths between 25–100 m, with N. helfrichi and N. lavandula rarely straying above 30 m. Putative hybrids identified based on intermediate colouration have been reported between N. magnifica and N. decora ( Fig. 8G View Fig ), and between N. magnifica and N. lavandula ( Fig. 8H View Fig ), although to the best of our knowledge no specimens have been made available for study. No hybrids have been reported for N. helfrichi , which shares its distribution range with N. magnifica .
Results from our molecular phylogenetic analyses support Springer’s proposed relationships, with N. decora recovered as sister to a lineage comprising N. helfrichi and N. lavandula (= N. helfrichi s.l. in Springer, 1982) (Fig. 11). We note, however, that both UFBS and SH-aLRT support values at this node were relatively low, and owing to the scarcity of N. helfrichi and N. lavandula specimens in museum collections, only one of each species were represented in our molecular dataset. Nemateleotris magnifica was recovered as the sister lineage to all other Nemateleotris . Despite the large geographical distributions of N. magnifica and N. decora , intraspecific variation for both species were generally lower than 1%, even for populations occurring in separate ocean basins (i.e., Pacific and Indian Ocean populations). While some individuals showed intraspecific variation greater than that between closely related sister species (such as N. helfrichi and N. lavandula ), they were not correlated with geographically distinct populations. In particular, N. decora from Pacific and Indian Ocean localities do not reflect reciprocally monophyletic groups, further justifying the synonymy of N. exquisita within N. decora . We however emphasise that the relationships inferred in this study are based only on mitochondrial COI, and a more robust dataset with more markers and increased sampling, especially for N. helfrichi and N. lavandula , is likely necessary to adequately assess the relationships between these widespread populations.
Identity of Zagadkogobius ourlazon Prokofiev. Prokofiev (2017) described Zagadkogobius ourlazon on the basis of the 18 mm SL holotype from south of the Anambas Islands in the Riau Archipelago, South China Sea. The specimen was trawled from a depth of 73 m. He placed his new species within the Ptereleotrinae based on it having a laterally compressed body, a lateral positioning of the eyes, five rays in the first dorsal fin, and unbranched rays in the second dorsal and anal fins. The presence of “five rays” (presumably V spines) in the first dorsal fin is unusual, as all seven of the recognised ptereleotrine genera (and indeed most gobiids) have six spines in the first dorsal fin. Additionally, Zagadkogobius is diagnosed in having very large scales, numbering 25 in lateral series (cycloid anteriorly, ctenoid midposteriorly), a filamentous first dorsal fin, a lanceolate caudal fin, and a coronal commissure connecting the median interorbital pore to the left and right oculoscapular canals. The holotype also possesses large scale pockets on the cheeks, upper portion of the opercle, and head. While some of these aforementioned characters occur within the ptereleotrines, the combination of them, as well as the presence of large scales on the body, cheeks, and head makes its identity as a species of ptereleotrine unlikely. These characters are more suggestive of species of Tryssogobius instead, which can appear ptereleotrine like due to having laterally compressed bodies, as well as a filamentous first dorsal fin and lanceolate caudal fin in some species. Notably, Tryssogobius is distinct among gobiids in having large scales on the cheek, opercle, and head, and in having a lateral series of 24–26 large scales ( Larson & Hoese, 2001). One species of Tryssogobius , T. quinquespinus , is known to possess five dorsal-fin spines ( Randall, 2006), an unusual condition for gobiids, and here shared with Zagadkogobius . Since we were unable to examine the holotype of Z. ourlazon , it is neither possible to confirm its validity nor placement within the Gobiidae (at the generic or phylogenetic level). We do however consider the exclusion of Zagadkogobius as a member of the Ptereleotrinae justified, at least based on consideration of the aforementioned characters.
Material examined. Nemateleotris decora (n=7): AMS I.16877-007, paratype, 37.1 mm SL, Osprey Reef , Coral Sea ; AMS I.17500-017, 57.4 mm SL, Tanavulu Point, Florida Island, Solomon Islands ; AMS I.17530-004, 50.9 mm SL, Alite Reef , off Malaita, Solomon Islands ; AMS I.17504-007, 45.7 mm SL, Cape Tawui, off Rabaul, New Britain; AMS I.45300-166, 33.5 mm SL, cleared and stained specimen from the aquarium trade; CAS-ICH 47426, (specimen almost broken in half, approximately 43.2 mm SL), Tabuaeran Atoll , Line Islands, Kiribati ; ZRC 47158, 51.3 mm SL, Bali, Indonesia ; Nemateleotris exquisita (n=7): BPBM 21528, paratype, 50.6 mm SL, Ras Muhammed, Red Sea; BPBM 22544, paratype, 61.3 mm SL, west coast off Flic en Flac , Mauritius ; CAS-ICH 234071, paratype, 53.0 mm SL, off Flic en Flac , Mauritius ; SAIAB 185925, paratype, 46.5 mm SL, Sodwana Bay , Kwazulu-Natal, South Africa ; SAIAB 186384, 3, paratypes, 55.0– 63.1 mm SL, Sodwana Bay , Kwazulu-Natal, South Africa ; Nemateleotris helfrichi (n=5): BPBM 11595, holotype, 43.3 mm SL, Tahiti , Society Islands; BPBM 11598, paratype, 49 mm SL , same data as holotype; BPBM 13095, paratype, 32.9 mm SL, Rurutu , Austral Islands; BPBM 13326, paratype, 40.8 mm SL, Takaroa, Taumotu Archipelago; ZRC 61811, 62.4 mm SL, Cook Islands ; Nemateleotris magnifica (n=8): AMS I.22583-035, 28.9 mm SL, Escape Reef , Australia ; AMS I.25110-035, 46.9 mm SL, Osprey Reef, Coral Sea; AMS I.25112-048, 2, 38.2–40.3 mm SL, Osprey Reef , Coral Sea; AMS I.39012-014, 35.6 mm SL, Santa Cruz Island, Solomon Islands ; AMS I.46486-050, 35.0 mm SL, Morane , Tuamotu Archipelago, French Polynesia ; AMS I.45300-327, 40.5 mm SL, cleared and stained specimen from the aquarium trade; ZRC 47157, 36.6 mm SL, Bali, Indonesia .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nemateleotris lavandula
| Tea, Yi-Kai & Larson, Helen K. 2023 |
Nemateleotris helfrichi
| Randall & Allen 1973 |
N. helfrichi
| Randall & Allen 1973 |