Lebbeus sokhobio, Marin, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.604 |
|
publication LSID |
lsid:zoobank.org:pub:7F2F71AA-4282-477C-9D6A-4C5FB417259D |
|
DOI |
https://doi.org/10.5281/zenodo.3671433 |
|
persistent identifier |
https://treatment.plazi.org/id/71124376-1465-4956-8ADF-ED19380E78D6 |
|
taxon LSID |
lsid:zoobank.org:act:71124376-1465-4956-8ADF-ED19380E78D6 |
|
treatment provided by |
Plazi |
|
scientific name |
Lebbeus sokhobio |
| status |
sp. nov. |
Lebbeus sokhobio sp. nov.
urn:lsid:zoobank.org:pub:7F2F71AA-4282-477C-9D6A-4C5FB417259D
Figs 1–6 View Fig View Fig View Fig View Fig View Fig View Fig
Lebbeus sp. – Marin 2018: 331.
Type material
Holotype GoogleMaps
SEA OF OKHOTSK • ♀; NE slope of Kuril Basin, st. 1-10 GoogleMaps ; 46˚08.9΄ N, 145˚59.4΄ E– 46˚09.0΄ N, 145˚59.5΄ E; depth 3303–3308 m; 10 Jul. 2015; ZMMU Ma5836 .
Paratypes
SEA OF OKHOTSK • 1 ♂; same collection data as for holotype; ZMMU Ma6096 • 1 ♀; NE slope of Kuril Basin, st. 4-3; 47˚14.0΄ N, 149˚34.8΄ E; AGT; depth 3366 m; 16 Jul. 2015; ZMMU Ma6097 GoogleMaps • 1 ♀, 1 ♂; NE slope of Kuril Basin, st. 4-9; 47˚13.6΄ N, 149˚39.2΄ E; EBS; depth 3365 m; 16 Jul. 2015; SMF 51579 GoogleMaps • 2 ♂♂, 1 juv.; NE slope of Kuril Basin, st. 4-10; 47˚12.2΄ N, 149˚36.7΄ E; EBS; depth 3366 m; 17 Jul. 2015; MIMB 39426 View Materials GoogleMaps • 1 ♀; NE slope of Kuril Basin, st. 7-12; 46˚54.6΄ N, 151˚03.7΄ E; AGT; depth 3301 m; 22 Jul. 2015; MIMB 39427 View Materials GoogleMaps .
Etymology
This new species is named after the SokhoBio (Sea of Okhotsk Biodiversity Study) Expedition 2015, which allowed the collection of numerous deep-sea species such as this one.
Description
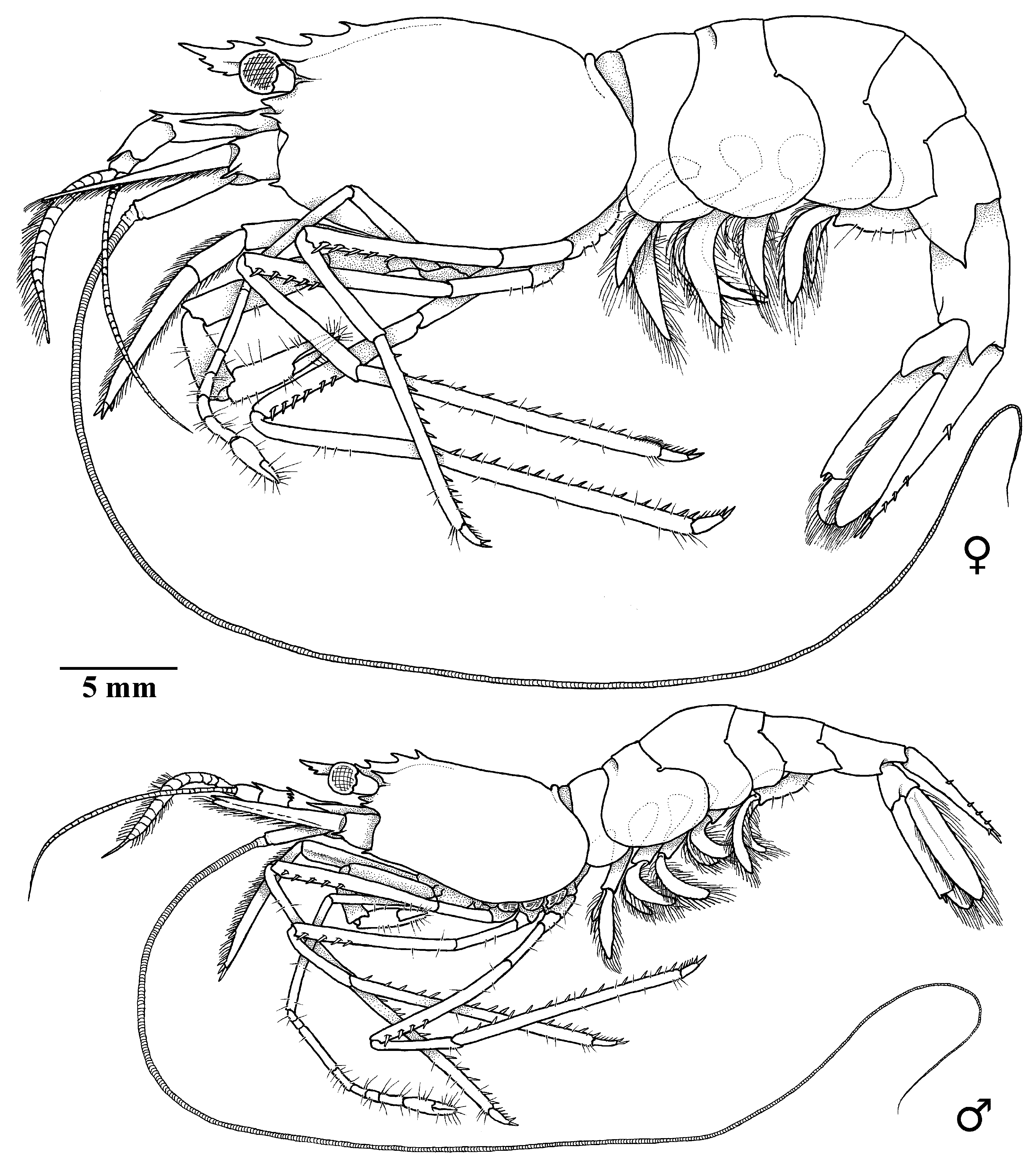
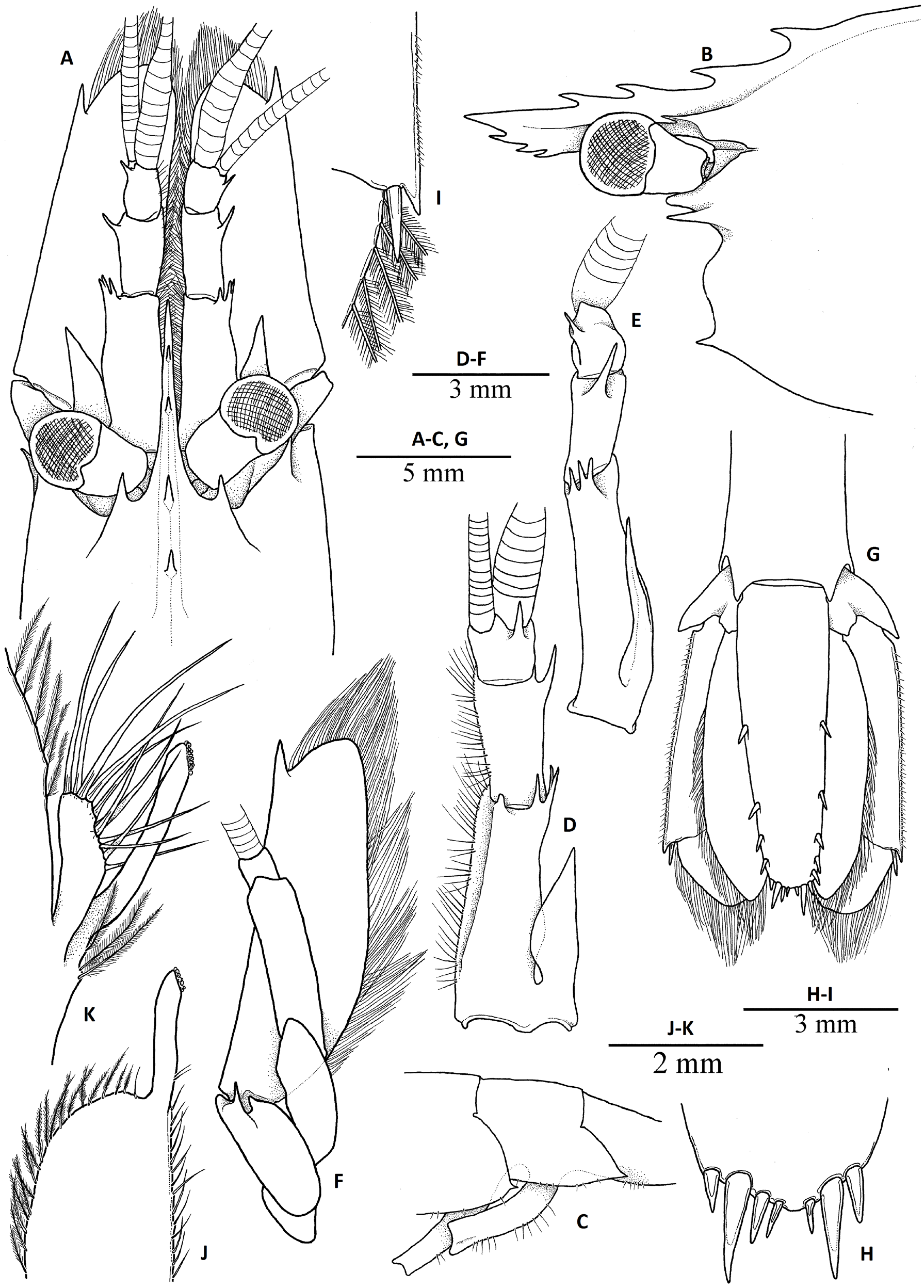
CARAPACE ( Figs 2 View Fig , 3 View Fig A–B). Smooth, without setae; dorsal surface slightly convex in males and gibbous in females, with well-marked postrostral median ridge armed with 2 postrostral teeth located at about anterior 0.2 of carapace length ( Fig. 5 View Fig ); antennal tooth situated slightly below suborbital angle ( Figs 3 View Fig A–B, 5); supraorbital tooth large, directed forward, with deep notch below base, situated anterior rostral base; suborbital lobe prominent, triangular; anterolateral margin between antennal and pterygostomial teeth strongly sinuous, with deep concavity below antennal tooth; pterygostomial tooth acute, smaller and more slender than antennal and supraorbital teeth, overreaching anterior margin of carapace ( Fig. 3 View Fig A–B).
ORBITS. Well developed, orbital margin with slight convexity posteriorly, base of eyestalk located between this convexity and suborbital lobe.
ROSTRUM. Relatively long, compressed, reaching distal margin of basal segment of antennular peduncle ( Fig. 3 View Fig A–B), about 0.3 times as long as carapace; rostral formula 1–2+2/1–3 ( Fig. 5 View Fig ), with welldeveloped dorsal and ventral lamina ; lateral rostral carina obsolescent, situated above level of proximal orbital margin ( Fig. 2A View Fig ).
PLEON ( Figs. 2 View Fig , 3C View Fig ). Smooth and unarmed dorsally; pleomere II with distinct anterior transverse groove on tergum; pleurae of pleomeres I–IV rounded, pleurae of pleomere IV pointed posteroventrally in some specimens ( Fig. 3C View Fig ); pleurae of pleomere V ( Fig. 3C View Fig ) with small posteroventral tooth; pleomere VI ( Fig. 3G View Fig ) with small posteroventral teeth and posterolateral process terminating acutely. Telson ( Fig. 3G View Fig ) slender, about 4 times as long as proximal width, narrowing posteriorly, with 3–5 (usually 4) pairs of small submarginal dorsal spines at 0.4, 0.75, 0.8 and 0.9 of telson length; posterior margin armed with 3 or 4 pairs (usually 4, but 3 pairs in possibly damaged specimens) of unequal spines or spiniform setae ( Fig. 3H View Fig ).
EYES ( Fig. 3 View Fig A–B). Normal, well developed, subpyriform, with subcylindrical eyestalk and large dilated cornea; eyestalk about as long as wide; cornea subglobose, without papilla; ocellus absent.
ANTENNULA. Antennular peduncle ( Fig. 3A View Fig , D–E) well developed; basal segment about twice as long as wide, with dorsodistal margin armed with 3 slender spines; stylocerite well developed, acute, nearly reaching distal margin of basal segment, mesial margin sinuous; intermediate segment (article 2) stout, about 1.5–2.0 times as long as wide, with slightly convex mesial margin bearing long plumose setae and slender distolateral tooth; distal segment (article 3) short, about as long as wide, about half the length of intermediate segment, with acute dorsolateral subdistal tooth, with long plumose setae along mesial margin; upper antennular flagellum with aesthetasc-bearing portion consisting of 10–12 articles. No sexual dimorphism detected.
ANTENNA ( Fig. 3F View Fig ). Normal, well developed; basicerite armed with small tooth ventrolaterally; carpocerite overreaching midlength of scaphocerite; flagellum well developed; scaphocerite wide, greatly overreaching antennular peduncle, about 3 times as long as maximal width, with well-developed distolateral tooth reaching distal margin of blade.
MOUTHPARTS. Typical for genus, without distinctive features. Mandible with 2-segmented palp; incisor process well-marked, terminating in sharp tip, bearing 4 distinct teeth and several additional denticles; molar process terminating distally. Maxilla I consisting of well-developed and partly fused endites, armed with spiniform setae and unsegmented bilobed palp. Maxilla II with simple, slender blunt palp; upper endite bilobed, fringed with setae; lower endite reduced; scaphognathite well developed, with rounded posterior lobe. Maxilliped I with partly fused endites, bearing short stout setae along distal margin as well as some elongated setose setae along distodorsal angle; exopod well developed, with well-marked caridean lobe with many setae; palp 2-segmented; epipod ear-shaped, bilobed distally. Maxilliped II with well-developed exopod, fringed with setae distally; ischium stout, with long setae along lateral margin; propodus short, length equal to that of dactylus, with convex dorsal margin furnished with long simple setae, ventral margin unarmed; dactylus convex, armed with numerous stout, long, simple setae along distal margin; exopod flagellate; epipod well-marked, distally bilobed, with podobranch. Maxilliped III ( Fig. 4 View Fig B–C) moderately long and stout, slightly overreaching scaphocerite and antennular peduncle; epipod well developed; exopod absent; antepenultimate article about 6 times as long as wide, slightly tapering distally, with longitudinal row of long spiniform setae along lateral surface and 3 long spiniform setae on distal margin; penultimate article about twice as long as wide, smooth; terminal segment about 7 times as long as wide, with distal margin oblique, armed with row of spines along distomesial margin.
PEREIOPOD I ( Fig. 4D View Fig ). Moderately robust; coxa with epipod and setobranch; basis stout, unarmed; ischium stout, about twice as long as wide, with long, simple setae along ventral margin; merus slender, about 4 times as long as wide, with row of spiniform setae proximally ( Fig. 4F View Fig ); carpus robust, about half the length of merus and slightly shorter than propodus, about twice as long as wide, slightly flaring distally; distal margin slightly overlapping carpo-propodal articulation; mesial surface with grooming apparatus consisting of shallow concavity and complex of short, stiff setae; propodus (palm) about 3 times as long as wide, subcylindrical, smooth; fingers ( Fig. 4E View Fig ) stout, about half the length of palm, subspatulate, about as long as wide; cutting edges straight, with strong distal teeth.
PEREIOPOD II ( Fig. 4G View Fig ). Relatively slender, unarmed; coxa with setobranch and epipod; basis small, about as long as wide; ischium about 4 times as long as wide, smooth; merus about 7 times as long as wide; carpus subdivided into 7 sub-articles with ratio of about 1:1:4:2:1:1:2; propodus (palm) subcylindrical, slightly shorter than distal carpal segment, about twice as long as wide and twice as long as fingers, with straight smooth margins; fingers slender, about 1.5 times as long as wide, with straight cutting edges.
PEREIOPODS III–V. Similar, relatively slender. Pereiopod III ( Fig. 4H View Fig ) coxa with setobranch and terminally hooked epipod; basis with small lobe distoventrally, about as long as wide; ischium about 3.5–4.0 times as long as wide; merus about 9 times as long as wide, armed with 5–8 movable spines on lateral surface adjacent to ventral margin on distal 4/5; carpus about 6 times as long as maximal width, slightly widened distally; propodus about 10–11 times as long as wide, with straight margins, ventral margin armed with tooth-like setae; dactylus ( Fig. 4J View Fig ) slender, terminating in elongate curved unguis, with 6–7 small accessory spinules, increasing in length distally. Pereiopods IV ( Fig. 4K View Fig ) and V ( Fig. 4M View Fig ) without epipod; merus of pereiopod V ( Fig. 4L View Fig ) with a single spine subterminally; propodus of pereiopod V with brush-like cluster of setae ( Fig. 4M View Fig ) (grooming apparatus) on flexor margin distally.
PLEOPODS. Endopod of pleopod I with terminally located appendix interna and row of curved spinulelike setae along mesial margin in males ( Fig. 3K View Fig ). Pleopod II in males with appendix masculina shorter than appendix interna ( Fig. 3J View Fig ), truncate terminally, bearing 10–12 long simple setae on distal and mesial surfaces. Uropods moderately slender, exceeding telson ( Fig. 3G View Fig ); distolateral margin of exopod with fixed posterolateral tooth and slender mobile spine (spiniform seta).
COLORATION. Body and appendages entirely vermilion, antennular and antennal flagella white; corneas of eyes with golden reflection ( Fig. 6 View Fig ).
SIZE. Largest female (holotype) has pcl. 19.0 mm and tl. 62 mm. Largest male has pcl. 17.0 mm and tl. 56 mm.
Remarks
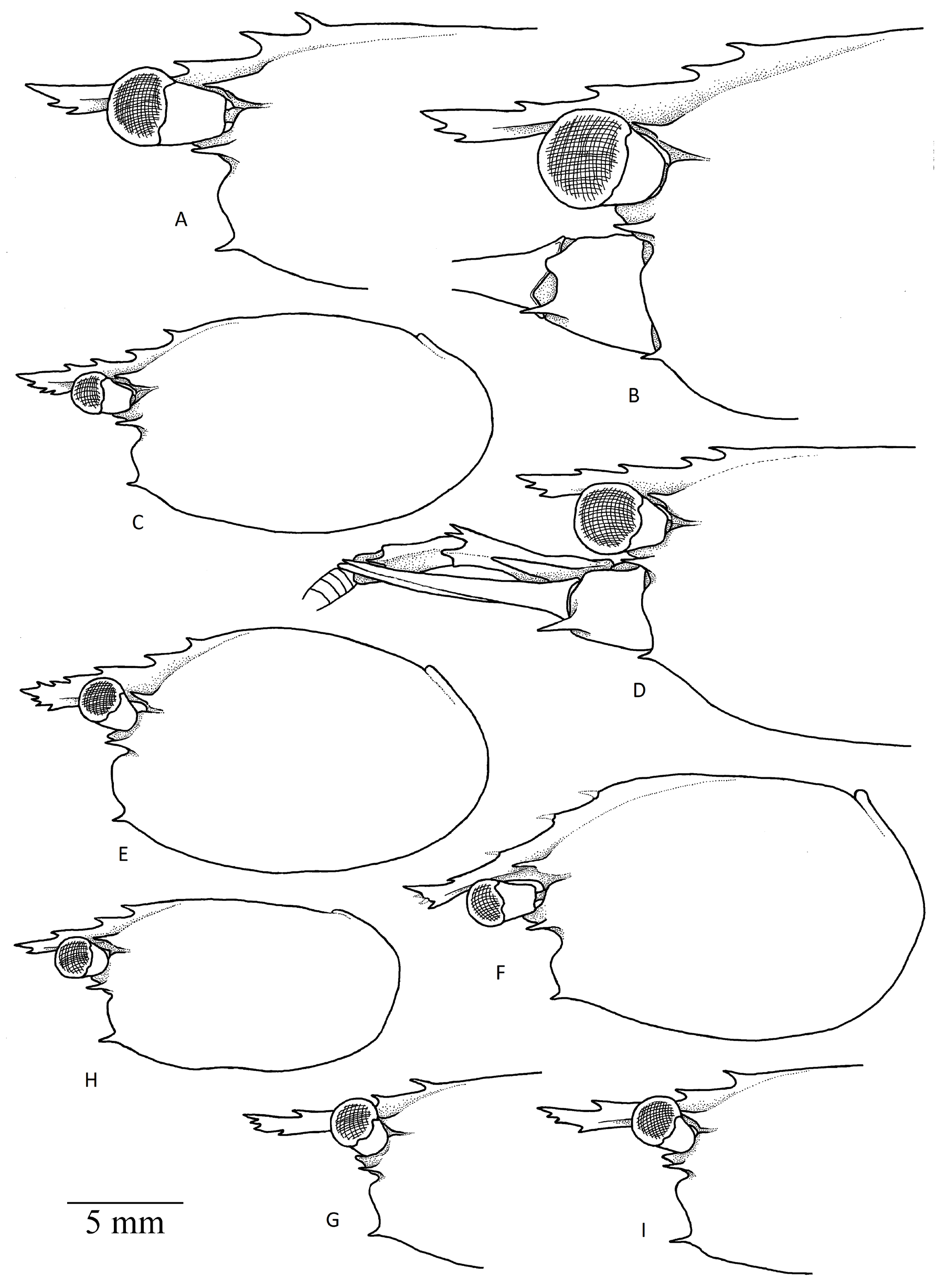
The new species described here belongs to the species group of the genus Lebbeus characterized by the presence of strap-like epipods on maxilliped III and pereiopod III. This species group includes L. africanus Fransen, 1997 , L. antarcticus ( Hale, 1941) , L. bidentatus Zarenkov, 1976 , L. brevirostris Chang et al., 2010 (described based on a possibly juvenile specimen), L. carinatus Zarenkov, 1976 , L. cristatus Ahyong, 2010 , L. formosus Chang et al., 2010 , L. indicus Holthuis, 1947 (questionable), L. java Komai et al., 2019 , L. kuboi Hayashi, 1992 , L. lamina Komai, 2013 , L. laurentae Wicksten, 2010 , L. microceros (Krøyer, 1841) , L. pacmanus Komai et al., 2012 , L. polyacanthus Komai et al., 2004 , L. profundus (Rathbun, 1906) , L. saldanhae (Barnard, 1947) , L. shinkaiae Komai et al., 2012 , L. similior Komai & Komatsu, 2009 , L. thermophilus Komai et al., 2012 , L. tosaensis Hanamura & Abe, 2003 , L. unguiculatus Chang et al., 2010 , L. vicinus ( Rathbun, 1902) , L. virentova Nye et al., 2013 , L. washingtonianus ( Rathbun, 1902) and L. wera Ahyong, 2009 . The wide geographical distribution and isolation of some deep regions (e.g., Antarctica vs the Sea of Okhotsk, etc.) suggests the hypothesis that numerous endemic deep water species are present within this group (e.g., Hayashi 1992; Komai et al. 2004, 2012, 2019; Komai 2013, 2015; Anosov et al. 2018). Lebbeus sokhobio sp. nov. is the only species of this group found in the northern part of the NW Pacific, the Sea of Okhotsk. The geographically closest species is L. lamina described from deep waters off the Izu Islands, Japan.
At the same time, the phylogenetic significance of grouping based on marked morphological features is rather doubtful ( Komai et al. 2019) and has not yet been proven due to the lack of sufficient genetic data (see below). However, based on the presence of epipods on the basis of pereiopods I–III, the shape and armament of telson, with 4 pairs of dorsal spines, and the relatively slender dactyli of the ambulatory pereiopods, armed with numerous small accessory spinules, the new species may be close to L. africanus from Mauritania, at a depth of 1500 m ( Fransen 1997), L. antarcticus from the South Ocean, at depths of 450–1775 m ( Nye et al. 2013b), L. bidentatus known from off Chile, at a depth of 1680 m ( Zarenkov 1976; Fransen 1997), L. carinatus collected off Peru, at depths of 1680–1860 m ( Zarenkov 1976; Fransen 1997), L. cristatus from New Zealand, at depths of 1231– 1226 m ( Ahyong 2010), L. formosus from Taiwan, at depths of 635–1982 m ( Chang et al. 2010), L. java from south of Java, at depths of 637–689 m ( Komai et al. 2019), L. saldanhae collected along the coasts of South Africa (Saldanha Bay), at a depth of about 265 m (145 fms) ( Fransen 1997), L. similior from Japan, at a depth of 1196 m ( Komai & Komatsu 2009), L. unguiculatus from Taiwan and Japan, at depths of 742–1262 m ( Chang et al. 2010; Komai 2011), L. vicinus known from along the Pacific coasts of North America from Alaska to Mexico, at depths of 954–2824 m ( Rathbun 1902, 1904; Wicksten & Mendez 1982) and L. virentova from the Mid-Cayman Spreading Center, Caribbean Sea, at a depth of 2294–2375 m ( Nye et al. 2013a). The morphological features shared among these species include: short styliform rostrum, not reaching distal margin of second antennular segment, armed with 4 or more dorsal teeth, including postrostral teeth and with more than 1 ventral tooth; distinct U- or V-shaped notch inferior to base of supraorbital tooth; sinuous anterolateral margin of carapace between antennal and pterygostomial teeth with deep excavation below antennal tooth; pleomere II with distinct anterior transverse groove on tergum; basal antennular segment with 2 or 3 dorsodistal teeth; dactyli of ambulatory pereiopods armed with accessory spiniform spinules over entire length of flexor margin (after Nye et al. 2012, with some modifications).
Some morphological features allow the separation of L. sokhobio sp. nov. from some of the other species in this group mentioned above. The new species is distinguishable from L. java by the different rostral formula and the presence of 4 (2+2) dorsal rostral teeth (vs 3 (2+1) in L. java ); the presence of 4 or 5 pairs of dorsal spines on the telson (vs 3 in L. java ); inner distal spines on the telson much shorter than those in L. java ( Komai et al. 2019: fig. 2e); shorter stylocerite of the basal antennular segment not reaching the distal margin of the segment (vs reaching the distal margin in L. java ; Komai et al. 2019: fig. 2b); distal part of penultimate article of maxilliped III ( Fig. 4A View Fig ) with fewer but more slender spines than in L. java ( Komai et al. 2019: figs 2g, 3a); merus of pereiopod III armed with 6–9 movable teeth at the distal angle (vs a maximum of 5 in L. java ); and the different number of lateral spines on the meri of pereiopods III–V (see Fig. 4 View Fig F–G, I–K vs Komai et al. 2019: figs 3e–f).
Lebbeus virentova can be separated from the new species by its shorter rostrum, with 3 postrostral teeth ( Nye et al. 2013a: fig. 2a–b) (vs rostrum significantly overreaching cornea, with only 2 postrostral teeth in L. sokhobio sp. nov.; Figs 3B View Fig , 5 View Fig ), the presence of 3 well marked dorsal rostral teeth ( Nye et al. 2012: fig. 2b) (vs only 2 teeth in the new species; Figs 3B View Fig , 5 View Fig ), and its white coloration ( Nye et al. 2013a: fig. 5) (vs vermillion coloration in the new species; Fig. 6 View Fig ). Moreover, L. virentova is known only from the Caribbean Sea.
Another very geographically distant species, L. laurentae , although rather poorly described, can be separated from L. sokhobio sp. nov. by the more slender distal part of the rostrum and its feebly marked dorsal and ventral armature ( Wicksten 2010; Komai et al. 2012) in contrast to the rostrum of the new species, which has well-marked dorsal teeth and some extension in the distal part, with well-developed ventral teeth ( Figs 3B View Fig , 5 View Fig ).
Lebbeus sokhobio sp. nov. can be separated from L. antarcticus by the more slender distal part of the rostrum ( Nye et al. 2013b: fig. 8b) (vs rostrum with some extension in the distal part, with well-developed ventral teeth in the new species; Figs 3B View Fig , 5 View Fig ) and the presence of 3 postrostral teeth (see Nye et al. 2013b: fig. 8b) (vs 2 in the new species).
Lebbeus cristatus and L. formosus differ from the new species in having a more slender and short rostrum ( Ahyong 2010: fig. 1a–c; Chang et al. 2010: fig. 4a–b), a different armature of the distal margin of the basal antennular segment ( Ahyong 2010: fig. 1d; Chang et al. 2010: fig. 4a–b) and of the posterior margin of the telson ( Ahyong 2010: fig. 1g; Chang et al. 2010: fig. 4e), and a smaller number of lateral spines on the meri of pereiopods III–V ( Ahyong 2010: fig. 1d–g; Chang et al. 2010: fig. 5e, g–h).
Lebbeus lamina and L. unguiculatus can also be clearly separated from the new species. Lebbeus lamina can be separated by its shorter rostrum and 3 postrostral dorsal teeth (vs only 2 in the new species), 7 pairs of small dorsal sublateral spines (vs only 4 pairs of relatively long spines in the new species), a different armature of the posterior margin of the telson (5–6 pairs of distal spines in L. lamina vs 4 in the new species), a smaller number of meral spines (4 in L. lamina vs 5–9 in L. sokhobio sp. nov.) and stouter dactyli of pereiopods III–V (after Komai 2013). Lebbeus unguiculatus differs in having a shorter rostrum and longer stylocerite, rounded pleura of pleonite IV (vs pointed in the new species), a different armature of the posterior margin of the telson (5 pairs of distal spines in L. unguiculatus vs 4 in the new species) and fewer lateral spines on the meri of pereiopods III–V (after Chang et al. 2010).
Genbank accession numbers
COI: MN590012 View Materials (holotype), MN590013 View Materials – MN590015 View Materials , MN608153 View Materials – MN608155 View Materials .
Genetic differences
The intraspecific pairwise genetic distances (p -distances) within the studied population of Lebbeus sokhobio sp. nov. (n=7) is 0.004 ±0.002 (d ±ES), which is rather low. Also, the genetic differences between specimens from different stations and the intraspecific differences among specimens from one station are very similar. Genetic p -distances between known species of the genus vary from 0.014 to 0.16 substitutions per 100 nucleotide positions (see Table 2 View Table 2 ), showing that the interspecific genetic differences of closely related species from different, sometimes very distant, regions of the World Ocean (e.g., L. antarcticus , L. virentova and L. sokhobio sp. nov.; see Fig. 7 View Fig ) are only slightly different from the intraspecific differences within the Kuril Basin of the Sea of Okhotsk. Unfortunately, much genetic data from genetic markers other than COI mtDNA are not currently available. However, it is very interesting that the genetically closest (= phylogenetically related) species among representatives of the genus Lebbeus are distributed most distantly – L. antarcticus from the Southern Ocean and L. virentova from the Caribbean (see Fig. 7 View Fig ). The genetic p -distances ( Table 2 View Table 2 ) between these species are lower than previously documented for caridean shrimps ( Knowlton et al. 1993; Knowlton & Weigt 1998; Hebert et al. 2003; Sites & Marshall 2004; Zakšek et al. 2007; Lefébure et al. 2006a, 2006b; Marin 2017).
At the same time, available barcoding data show that all of the deepest dwelling species belong to the same phylogenetic clade (see Fig. 7 View Fig ; ‘Deep-sea Lebbeus ’ clade) showing a low level of divergence among species, whereas their species are very widely distributed. Similar small interspecific distances of about 1–2% are also known from very distantly living species of other deep-sea caridean shrimps (e.g., Mirocaris Vereshchaka, 1997 (Alvinocarididae) ; Shank et al. 1999; Vereshchaka et al. 2015; data from GenBank), as well as other deep-sea invertebrate taxa such as bivalve mollusks (e.g., Abyssogena Krylova et al., 2010 (Vesicomyidae) ; Liu & Zhang 2018) and octocorals ( France & Hoover 2002). Low interspecific genetic differences in COI mtDNA were observed exclusively in deep-sea taxa, but, for example, not in all studied deep-sea caridean shrimps (e.g., Shank et al. 1999; Vereshchaka et al. 2015; Zhang et al. 2017). As suggested by France & Hoover (2002), possible explanations for such reduced rates of divergence include a lower rate of evolution for octocoral mitochondrial genomes (also supported by Shearer et al. 2002) and the presence of a gene, mtMSH, which may code for a mitochondrial DNA mismatch-repair system ( Culligan et al. 2000). The purpose of this study is not to try to answer the question of why the interspecific distances of the deep-sea clade within the genus Lebbeus as so low, given the small amount of genetic data available, but it can be concluded that interactions (= gene flow) between populations from the Sea of Okhotsk, the Caribbean and Antarctica are more difficult to imagine than to assume the presence of some mechanism interfering with the standard rates of evolution in the COI mtDNA gene of deep-sea species. In addition, in closely related shallow-water taxa, such as the widely distributed and abundant Thor amboinensis (De Man, 1888) (Thoridae) , geographic variability is well reflected in genetic changes ( Fig.7 View Fig ; Titus et al. 2018 for T. amboinensis ). The Canadian clade of Lebbeus polaris (Sabine, 1824) (see Fig. 7 View Fig ) shows a higher degree of COI mtDNA variability than deep-sea Lebbeus species from different regions of the world. Perhaps the use of other gene markers will allow deep-sea species to be divided more clearly, using molecular genetic methods, but at the moment there is an insufficient amount of genetic information for comparison in international depositories (e.g., GenBank (NCBI) database).
Distribution
Lebbeus sokhobio sp. nov. is so far known only from the Kuril Basin of the Sea of Okhotsk and is probably endemic for this region in accordance with current knowledge of the limited geographical ranges of species in the genus Lebbeus (e.g., Hayashi 1992; Komai et al. 2004, 2012; Komai 2015; Anosov et al. 2018). In the same bathymetric range along the neighboring Kuril-Kamchatka Trench and the adjacent abyssal plain of the northwestern Pacific, no specimens of this genus were collected, neither during earlier expeditions to the area or as the result of the more recent deep-sea trawling of the KuramBio I–II (Kuril-Kamchatka Biodiversity Studies) Expeditions ( Brandt & Malyutina 2012; Brandt et al. 2016; Malyutina et al. 2018; pers. obs.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lebbeus sokhobio
| Marin, Ivan 2020 |
Lebbeus
| Marin I. 2018: 331 |