Nototriton costaricense, Arias & Kubicki, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4369.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:583E62DF-CF37-449F-A65F-D6E60DE9991E |
|
DOI |
https://doi.org/10.5281/zenodo.5963669 |
|
persistent identifier |
https://treatment.plazi.org/id/0421277A-8B3B-B541-9DE9-62FEFB5852F5 |
|
treatment provided by |
Plazi |
|
scientific name |
Nototriton costaricense |
| status |
sp. nov. |
Nototriton costaricense View in CoL sp. nov.
Southern Moss Salamander
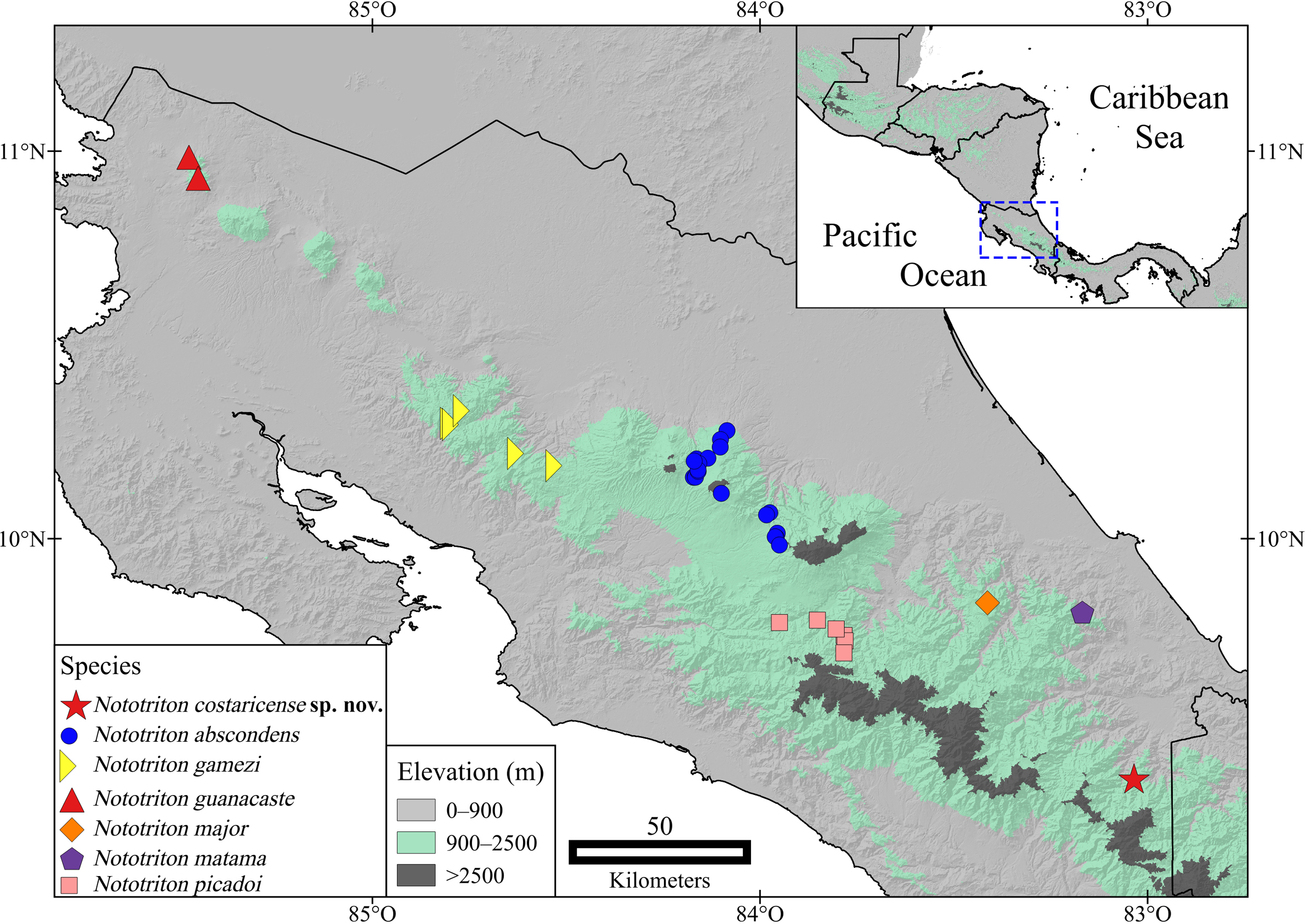
( Figures 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Holotype. UCR 22900 , a subadult from Costa Rica: Provincia de Limón: Cantón de Talamanca: Distrito de Telire : Cerro Pat , Parque Internacional La Amistad , ca. 1500 m a.s.l., obtained by Erick Arias on 11 March 2015.
Generic Placement. Assigned to the genus Nototriton due to having fewer than 14 costal grooves and reduced manus and pes that are longer than wide, and to the subgenus Nototriton based the molecular evidence presented herein.
Diagnosis. The combination of the following characteristics can be used to distinguish Nototriton costaricense from other described species in the subgenus Nototriton : (1) having at least the distal phalanges on fingers II, III, and IV in addition to the distal phalanges on toes II, III, IV, and V free of webbing; (2) large nostril openings, greater than 0.3 mm in diameter ( Fig. 5 View FIGURE 5 ); (3) robust habitus, TW = 14.6 % of SL; (4) long hind limbs, HLL = 21.9 % of SL; (4) differentiated from other members of the genus Nototriton , subgenus Nototriton by its 16S, COI, and cyt b mtDNA distances.
Comparisons. Since Nototriton costaricense is only known to occur in Costa Rica and molecular evidence strongly supports it forming part of the Nototriton picadoi species group, phenotypic comparisons are presented here only with respect to members of that clade (i.e., N. abscondens , N. gamezi , N. guanacaste , N. major , N. matama , and N. picadoi ), which are all endemic to Costa Rica ( Boza-Oviedo et al. 2012; Townsend 2016).
Contrasting characteristics for Nototriton costaricense are presented in parentheses. Nototriton abscondens ( Taylor, 1948) can be distinguished from N. costaricense by having relatively smaller nostrils openings, 0.3–0.8 % of SL (RNW = 1.3 % of SL); relatively narrower head, 12–14 % of SL (broader head, HeW = 15.1 % of SL); slender trunk, TW 8–10 % of SL (thick trunk, TW 14.6 % of SL). Nototriton gamezi Good & Wake, 1993 has relatively shorter hind limbs, 16–17 % of SL (longer hind limb, HLL = 21.9 % of SL); narrower head, 13–14 % of SL (broader head, HeW = 15.1 % of SL); slender trunk, TW 8–10 % of SL (thick trunk, TW 14.6 % of SL). Nototriton guanacaste García-París & Wake, 2000 has relatively smaller nostril openings, 0.4–0.95 % of SL (RNW = 1.3 % of SL); relatively shorter hind limb, 17–20 % of SL (longer hind limb, HLL = 21.9 % of SL). Nototriton major Good & Wake, 1993 has relatively smaller nostrils openings, 0.3 % of SL (RNW = 1.3 % of SL); shorter snout-gular length, 17.7 % of SL (longer snout-gular length, LGFS = 21 % of SL); relatively narrower head, 11.3 % of SL (broader head, HeW = 15.1 % of SL); slender trunk, TW 9–10 % of SL (trunk robust, TW 14.6 % of SL); relatively shorter hind limb, 19.3 % of SL (longer hind limb, HLL = 21.9 % of SL); relatively shorter fore limb, 14.8 % of SL (longer front limb, FLL = 20.1 % of SL). Nototriton matama Boza-Oviedo et al., 2012 has relatively larger nostrils openings, 2 % of SL (RNW = 1.3 % of SL); pointed outer toe tips (rounded outer toe tips); relatively narrower head, 14 % of SL (broader head, HeW = 15.1 % of SL). Nototriton picadoi ( Stejneger, 1911) has a relatively narrower head, 12–14 % of SL (broader head, HeW = 15.1 % of SL); slender trunk, TW 10–11 % of SL (trunk robust, TW 14.6 % of SL); relatively shorter hind limb, 18–19 % of SL (longer hind limb, HLL = 21.9 % of SL); relatively shorter fore limb, 16.9–18.2 % of SL (longer front limb, FLL = 20.1 % of SL). Although Nototriton tapanti Good & Wake, 1993 is not a member of the N. picadoi species group, but we decided to include a phenotypic comparison here due to the fact that no DNA sequences of N. tapanti were available for molecular comparison with N. costaricense . Nototriton tapanti can easily be distinguished from N. costaricense by its syndactylous hands and feet (having at least the distal phalanges on fingers II, III, and IV in and toes II, III, IV, and V free of webbing); tips of free digits pointed and without subterminal pads (rounded tips and terminal pads present).
Description of holotype. Subadult having a SL of 21.9 mm. Head slightly wider than neck and shoulders (HeW 3.3 mm, NeW 3.0 mm, ShW 2.9 mm), with greatest width of head just posterior to the articulation of the jaws; snout raised anterodorsally, spadate to rounded in dorsal outline, and rounded in profile; snout relatively short (SnL 0.7 mm, 3.2 % of SL), with nearly terminal non-protruding large nostrils (LNH 0.34 mm, RNW 0.31 mm) directed anterolaterally; internarial area convex in dorsal outline. Eyes relatively large (EW = 142 % of SnL), weakly protruding beyond dorsal and ventral outline of head, directed anterolaterally, with a distinct suborbital groove. Top of head flat and smooth, tapering slightly toward anterior terminus, lacking contrasting interorbital or other dermal structures. Canthus rostralis weakly rounded; intercanthal area flat to slightly convex; and loreal region slightly concave. No obvious cirri (nasolabial protuberances), but nasolabial grooves weakly discernable on tip of snout; nasolabial grooves start at lateral margins of nares and extend ventrally, with a slight outward orientation, and terminate prior to reaching upper lip margin. Gular fold well-defined, starting on dorsolateral portion of neck, and wrapping around lateral section of head and crossing venter as a smooth anterior-oriented curve. Upper lip markedly protruding beyond edge of lower lip in ventral view; and no mental gland visible under skin of anterior intermandibular region. A very weakly discernible groove starts at posterior dorsolateral margin of orbit and extends dorsolaterally to anterior margin of gular fold. Pair of weak, but discernably raised parotoid glands present on dorsolateral margin of head between orbits and occiput.
Arms relatively long and slender (FLL = 4.4 mm, 20.1% of SL), without noticeable hypertrophied forearm compared to upper arm. Hands small and slender (HaL = 1.2 mm, 29.3% of VGS; HaW = 1.0 mm, 30.3 % of HeW). Fingers II, III, and IV relatively long (LF2 0.47 mm, LF3 0.47 mm) and robust with rounded tips; fingers II and IV nearly equal in width, but Finger III noticeably wider (WF3 0.22 mm); terminal pads poorly discernable, appearing to be present on ventral tips of fingers I, II, III, and IV; Finger I barely protruding beyond palmar tissue; webbing absent between fingers II and III and fingers III and IV. Palmar surface appearing to be smooth overall, but with an evident dermal crease extending transversally near proximal edge. Dorsal surfaces of hand with well defined interdigital grooves extending across metacarpal region. Relative lengths of fingers on right hand I <IV <II <III.
Legs relatively long and slender (HLL 4.8 mm, 21.9 % of SL), with little perceivable difference between thickness of upper and lower leg. Feet small and slender (FoL 1.6 mm, 39.0 % of VGS; FoW 1.4 mm, 34.1 % of VGS). Toes II, III, IV, and V relatively long and slender with rounded tips nearly equal in width (WT3 0.26 mm) and length, toes III and IV slightly longer than others (LT2 0.71 mm, LT3 0.54 mm); terminal pads poorly discernable, appearing to be present on ventral tips of toes I, II, III, IV, and V; Toe I barely protruding beyond plantar tissue; webbing absent between toes II, III, IV, and V. Plantar surface appearing to be smooth overall, but with an evident dermal crease extending transversally near proximal edge. Dorsal surfaces of foot with well defined interdigital grooves extending across metatarsal region. Relative lengths of toes on right foot I <V<IV <II <III.
Body subcylindrical (wider than high) in cross section, and relatively robust (TW = 3.2 mm). Between axilla and groin, 11 costal grooves visible, 13 if counting axillary and inguinal grooves; costal grooves most visible on ventral and lateral portions of body. Adpressed limbs separated by 5 costal folds; 12 costal folds between axilla and groin. Slight middorsal depression extends longitudinally along length of body, starting at base of head and becoming indiscernible on anterior portion of tail. Tail long, cylindrical in cross section, lacking discernable caudal grooves or constriction at base, and tapering evenly to a pointed terminus. Tail long, cylindrical in cross section, lacking an evident constriction at base, and evenly tapering to pointed terminus; at least 15 caudal grooves discernible on anterior 3/4ths of tail, no grooves discernible on posterior 1/4th of tail. Skin on surfaces of head, body, limbs, and tail smooth.
Coloration in life. The ground color of the dorsal surfaces of the head and trunk consisted of a mixture of pale earthy tones (Light Flesh Color [250], Light Pratt’s Rufous [71], and Flame Scarlet [73]). Secondary coloration on the dorsal surfaces of the head and trunk consisted of irregular dark markings (Marron [39] and Dark Grayish Brown [284]), grayish white dashes, and numerous very fine circular Marron (39) chromatophores. On the posterior half of the parotoid glands there was a pale flesh colored patch. On the center of the head, between the orbits and occiput, there was a concentration of dark (Dark Grayish Brown [284]) irregular markings. On the left parotoid gland there was a dark wedge shaped mark, appearing to be a scar.
The upper surfaces of the arms and legs have a Dark Grayish Brown (284) ground color. Secondary colors on the upper surfaces of the arms and legs are in the form of irregular spots either pearly white, Light Buff (2), Light Flesh Color (250), and Flame Scarlet (73). The hands and feet were a pale translucent gray.
The dorsal surface of the tails was a mottled mixture of Flame Scarlet (73), Light Flesh Color (250), and Light Buff (2). The dominant color of the dorsal surface of the tail was Flame Scarlet. Additionally, there were numerous white spots and dashes on the dorsal surface of the tail.
The colorations on the lateral surfaces of the body and tail were divided by a concentration of white markings or dashes that especially on the trunk formed what could be weakly interpreted as a longitudinal band. The inferior half of the lateral body and tail was Dark Grayish Brown (284) with scattered white markings. The superior half of the body and tail consisted of a mixture of earthy tones typical of the overall adjoining dorsal surfaces. The lateral surfaces of the head, between the orbits and the gular fold, were dominated by Flame Scarlet chromatophores (73); the mandibular region was dark gray with some scattered white markings or spots. The iris was a coppery-red with scattered dark spots and reticulation.
The ventral skin of the head, body, and limbs was pale brown to gray with a very fine dark gray to black reticulation. Numerous white spots and irregular markings were also scattered throughout the ventral surfaces of the head and body. On the ventrolateral surface of the head, between the nuchal groove and gular fold, there was a patch of flame Scarlet skin marginally wrapping onto the ventral surface. Overall, the tone of the gular region was noticeably paler than the rest of the ventral surfaces. The palmar and plantar surfaces were pale grayish-white. The ventral surface of the tail is similar in general appearance to that of the body, but the ground color is noticeably a darker tone of gray.
Coloration in ethanol. After nearly two years in ethanol (70%), the overall coloration of the holotype has darkened throughout and contains a mixture of four principal colors; Chestnut (30), Flame Scarlet (73), Raw Umber (280), and a pale fleshy gray
Measurements (in mm), tooth counts, limb interval, and percentages of the holotype. SL 21.9; Tal 25.0; TL 46.9; ShW 2.9; HeW 3.3; NeW 3.0; EW 1.1; SnL 0.7; JSL 2.5; LGFS 4.6; LNH 0.34; RNW 0.31; IND 0.7; NLP 0.4; ICD 1.6; HLL 4.8; FLL 4.4; TW 3.2; VGS 4.1; FSL 6.4; UHL 2.7; AGL 12.0; VL 1.4; HaW 1.0; HaL 1.2; LF2 0.47; LF3 0.47; WF3 0.22; FoW 1.4; FoL 1.6; LT2 0.71; LT3 0.54; WT3 0.26. Limb interval 5. Measurements in related percentages: VGS/SL 18.7 %; IND/HeW 21.2 %; AGL/SL 54.8 %; HeW/SL 15.1 %; Hew/AGL 27.5 %; SnL/ HeW 21.2 %; LNH/HeW 10.3 %; LNH/SL 1.6 %; RNW/HeW, 9.4 %; RNW/SL 1.4 %; HLL/SL 21.9 %; FLL/SL 20.1 %; HaL/VGS 29.3 %; FoL/VGS 39.0 %; Haw/HeW 30.3 %; FoW/HeW 42.4 %; LT3/HeW 16.4 %.
Etymology. The specific epithet refers to the Spanish word meaning Costa Rican, “ costaricense ”. The name represents the fact that the holotype and species was discovered in Costa Rica. Given the close proximity of the type locality to the Costa Rica-Panama border, we speculate that one day it may indeed be discovered within Panama as well.
Habitat and natural history observations. The holotype of Nototriton costaricense was discovered inside a moss mat that was growing approximately one meter above the ground on the trunk of a tree. The type locality is ca. 2.5 km SSW of a peak known as Cerro Pat; the general site is a relatively flat ridge, ranging from 1400–1500 m asl, which is covered by mature forest. Despite the elevation of the site typically being dominated by cloud forest with an abundance of bryophytes and epiphytes, it was nearly devoid of such floral conditions; bryophytes and epiphytes were uncommon there. The type locality is located on a ridgeline that extends out towards the north from the Kamuk massif. Just south of the type locality, the topography of the ridgeline leading towards the Kamuk massif drastically rises until reaching an unnamed peak with an elevation of approximately 1850 m asl. The unnamed peak and its slopes is covered in mature forest, and in contrast to that of the type locality, there is an abundance of bryophytes and other epiphytes covering the trees. Despite an intense search by EA through moss, bromeliads, and leaf litter, in what appeared to be more optimal habitat along the slopes of the unnamed peak, no additional salamanders were found.
Inasmuch as Nototriton costaricense is only known from a single specimen, any assumptions about its natural history are highly speculative. With that said, however, like other members of the Nototriton picadoi species group, it is probable that this species is prone to living within moss mats, both in terrestrial and arboreal situations, within cloud forest. All known Costa Rican species of Nototriton are known to principally inhabit moss, unlike congeners of the subgenus Bryotriton found in Nuclear Central America that principally known to inhabit microhabitats such as leaf litter, rotting logs, and bromeliads ( Townsend et al. 2010, 2011, 2013; Townsend 2016). Within another patch of moss on the same tree where the holotype of N. costaricense was found, an individual of Bolitoglossa minutula was also discovered ( Arias 2017). Other amphibian species found in sympatry with Nototriton costaricense are Craugastor podiciferus , C. aff. underwoodi, Diasporus hylaeformis, Incilius epioticus, Pristimantis caryophyllaceus, and P. pardalis.
Distribution. Nototriton costaricense is known from a single site within the Lower Montane Rain Forest life zone ( Holdridge 1967) along the mid-elevation slopes of the extreme southeastern Cordillera de Talamanca, within Parque Internacional La Amistad, in the vicinity of Cerro Pat, ca. 1500 m ( Fig. 1 View FIGURE 1 ). Further explorations and encounters with this species are needed before we will be able to further understand its potential range. We speculate that it is very possible that N. costaricense extends into Panama due to the proximity and relatively homogenous habitat lying between the type locality and the border (~ 11 kilometers straight line distance). Nototriton costaricense , if found to inhabit Panama, would represent the first species of this genus to extend beyond Costa Rica.
Discussion
With the discovery and description of Nototriton costaricense , the number of species known for this genus within Costa Rica is now elevated to nine, and the total diversity of salamanders for the tiny country 52. In the last decade 11 species (23.5% of total) of salamanders were described from Costa Rica ( Wake et al. 2007; García-París et al. 2008; Bolaños & Wake 2009; Boza-Oviedo et al. 2012; Kubicki 2016; Kubicki & Arias 2016), indicating that the inventory of salamanders from the country is incomplete. The bolitoglossines recently described from Costa Rica are only known from a few localities and relatively few specimens, and half of these species are known from the Caribbean slopes of Costa Rica. We would like to emphasize, again, that special attention needs to placed on improving the knowledge of the diversity of not only salamanders, but amphibians in general along the midelevation Caribbean slopes of the Cordillera de Talamanca of Costa Rica. The mid-elevation slopes along the Caribbean versant of Talamanca, due to the difficult terrain and over all limited access, harbor some of the poorest known and least explored forests within Costa Rica; further inventories should be viewed as a priority in this overall region.
With our discovery of Nototriton costaricense , the distribution of genus is now known to extend ~ 50 km to the south-southeast from the previously known southern limit of the genus, which was the type locality of N. matama on the southeast terminus of the Matama ridge, Limón Province, Costa Rica. Between the type localities of N. matama and N. costaricense , there is an abundance of suitable habitat for the genus, and this area needs to be surveyed further in attempt to learn more about the actual distributional boundaries between these two enigmatic and recently described taxa.
The genetic distances between Nototriton costaricense and the other members of the N. picadoi species group range from 1.8–2.4 % for 16S; this distance is relatively low in comparison to those suggested by Fouquet et al. (2007) for Neotropical frogs, in which the authors recommend a minimum of 3% 16S divergence be recognized as threshold to define candidate species. We recently commented that this threshold possibly underestimates the true diversity of Neotropical salamanders ( Kubicki & Arias 2016), and that each taxon should be carefully evaluated on an individual basis considering all the phenotypic, geographic, and molecular evidence at hand. Kubicki & Arias (2016) described a well-supported morphologically distinctive new species of Bolitoglossa that had genetic distances as low as 2.0 % (16S) among its closest related taxa. Bolitoglossa splendida Boza-Oviedo et al., 2012 is another example of salamander that is well recognized for its phenotypic uniqueness, but presents once again a relatively low (2.0 % 16S) genetic divergence from of its closest related congeners ( Boza-Oviedo et al. 2012). Within the genus Nototriton it has been previously published and justified that low genetic distances are known exist among well-recognized species, i.e. 1.9 % in 16S between N. barbouri and N. limnospectator ( Townsend 2016) . Rovito et al. (2013) used morphological and molecular (two mitochondrial and one nuclear genes) data to define the species within another genus of miniaturized salamanders in Mexico, Thorius . Rovito et al. (2013) showed that an integrative approach (i.e. morphological and molecular data) could provide robust support for complex groups, despite the fact that the 16S divergences among several species of Thorius were equal to or less than 2%. More studies evaluating the validity of species weakly supported with mitochondrial DNA are needed.
According to Rovito et al. (2015) the genus Nototriton arose ca. 10 Ma and due the Nearctic origin of Bolitoglossini (Shen et al. 2016) it is highly probable that the genus arose in Nuclear Central America and only recently dispersed to Isthmian Central America –affected possibly by their low dispersal potential. According to this scenario, we speculate that the species of Nototriton inhabiting Costa Rica are the result of a rapid diversification during the Pliocene-Pleistocene, which would explain the relatively low genetic distance among members of the subgenus Nototriton . Nicolas et al. (2012) used three mitochondrial genes (16S, COI, and cyt b) to identify species within of a group of Old World rodents that arose during the Pleistocene; they founded very low genetic distances between the described species (as low as 0.49 % in 16S and 2.94 % in COI). Although, there is not a minimal divergence value suggested to differentiate species for cyt b the divergence is known to be as low as 5 % between species that are well supported morphologically, i.e. B. aureogularis and B. robinsoni ( Boza-Oviedo et al. 2012) . Johns & Avice (1998) reviewed the cyt b distances among vertebrates and they found distances less than 5 % between different species of amphibians and reptiles of the same genus. It is also important mention that the use of a threshold for species delimitation has been criticized if it is not accompanied by other evidence such as morphological, behavioral or ecological characteristics ( Meyer & Paulay 2005; Clare et al. 2011). Padial & De la Riva (2011) pointed out that thresholds may be useful to identify groups of interest for further study, but minimum levels of divergence for certain traits cannot be defined as a prerequisite for species recognition under the evolutionary species concept.
Due to the relatively few sequences available for members of the subgenus Nototriton , details surrounding the phylogenetic relationships within this taxon are for the most part poorly known. The two species groups within the subgenus Nototriton are well supported (i.e. N. picadoi s.g. and N. richardi s.g.), but the relationships within each group are poorly understood, especially so with the N. richardi species group (i.e. N. richardi and N. tapanti ) for which sequences are only available for N. richardi . Nototriton tapanti is currently only known from the adult female holotype which was collected in 1986 and unfortunately no tissues from this specimen are available for genetic analysis ( Good & Wake 1993).
Nototriton costaricense was found to be the sister clade to all species of the N. picadoi species group that inhabit Costa Rica, however the support for that relationship is relatively weak. Additionally, the alpha-level distinction between N. picadoi and N. matama is weakly supported with a 0.6% genetic distance for 16S (Boza- Oviedo et al. 2012) in addition to phenotypic support that is questionable due to possible artifact of preservation or desiccation that might have taken place during the manipulation of the specimens of the type series of N. matama . We feel that the relationship not only between N. picadoi and N. matama requires a further detailed review, but those interspecific relationships among the members of the N. picadoi and N. richardi species groups as a whole, with an emphasis to include more localities, DNA data, ecological data, and phenotypic data.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.