Memphis smalli Riley & Dias, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5061.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:D9DABC44-0317-4A90-931C-6A3C7F88C0FF |
|
DOI |
https://doi.org/10.5281/zenodo.5705783 |
|
persistent identifier |
https://treatment.plazi.org/id/C3A7F294-FE41-4C3D-A397-9328C6A94B39 |
|
taxon LSID |
lsid:zoobank.org:act:C3A7F294-FE41-4C3D-A397-9328C6A94B39 |
|
treatment provided by |
Plazi |
|
scientific name |
Memphis smalli Riley & Dias |
| status |
sp. nov. |
Description of Memphis smalli Riley & Dias sp. nov.
urn:lsid:zoobank.org:act:C3A7F294-FE41-4C3D-A397-9328C6A94B39
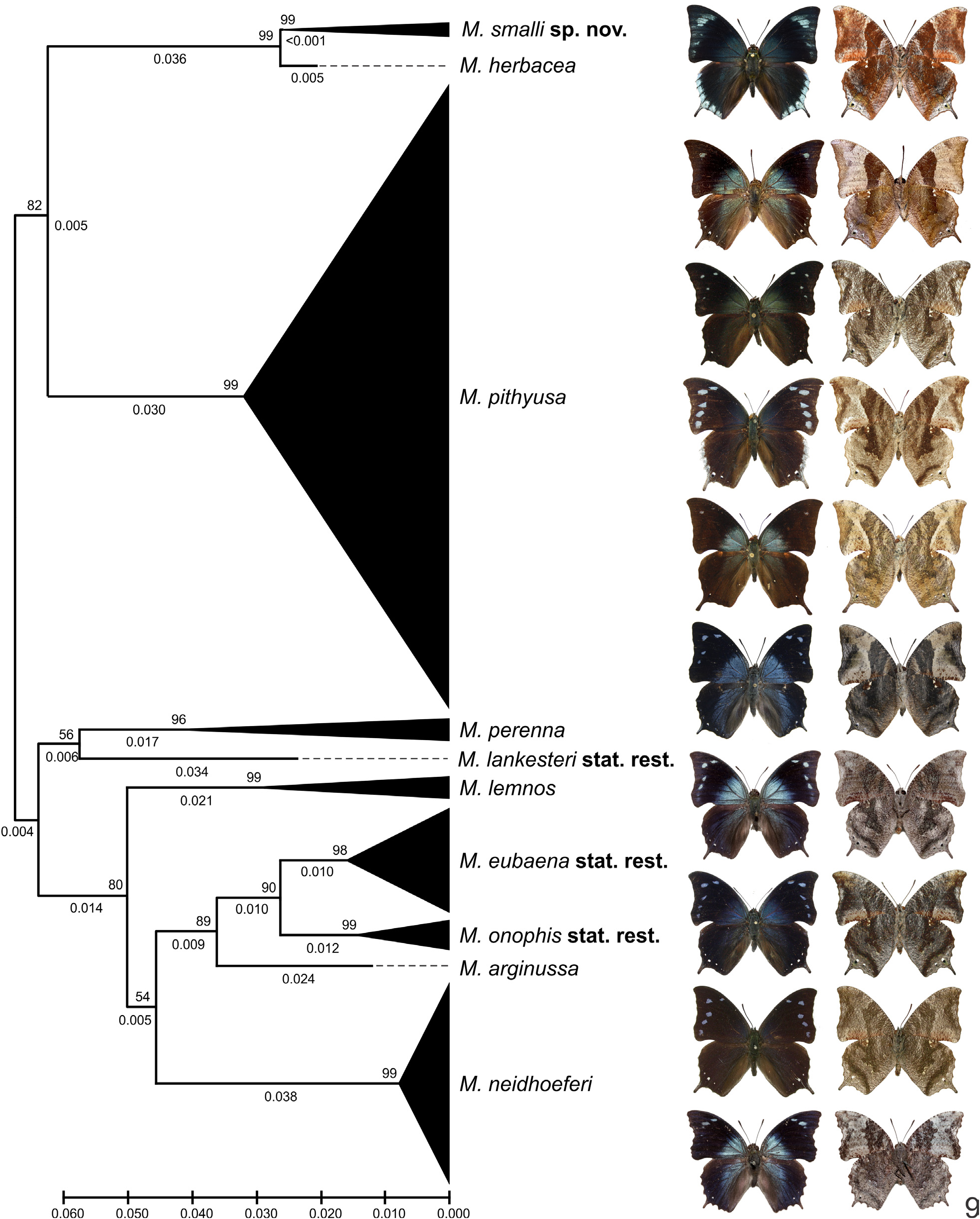
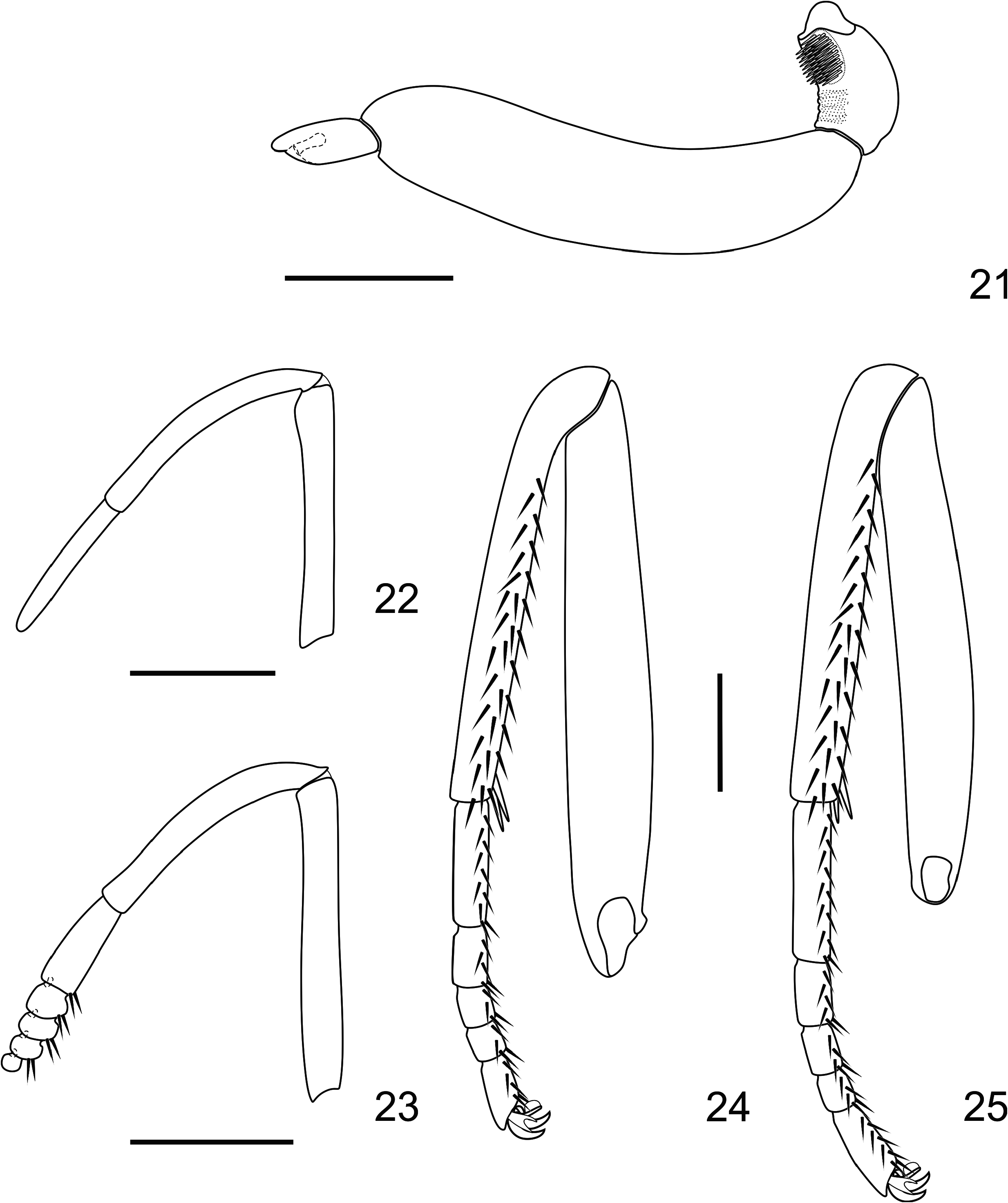
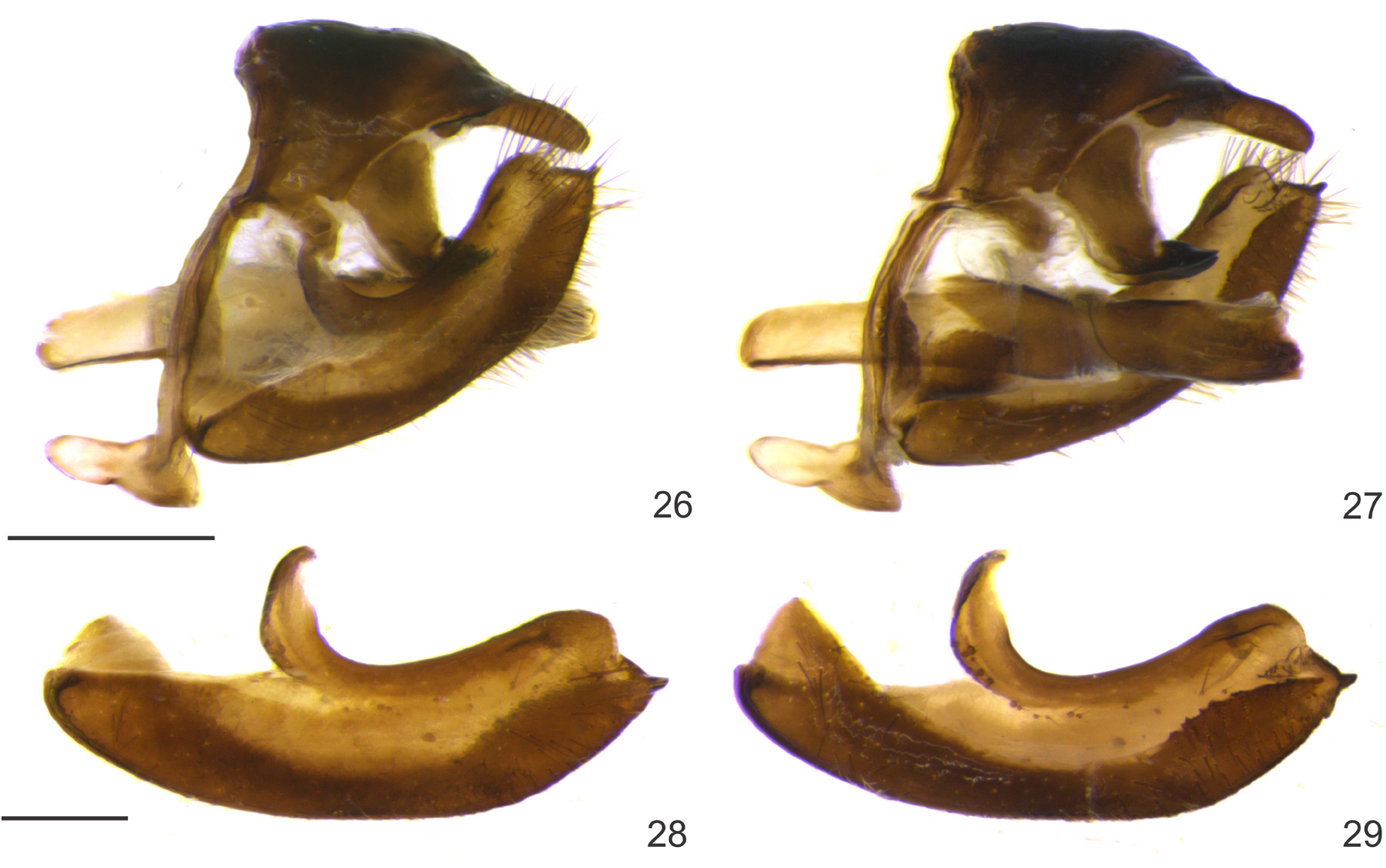
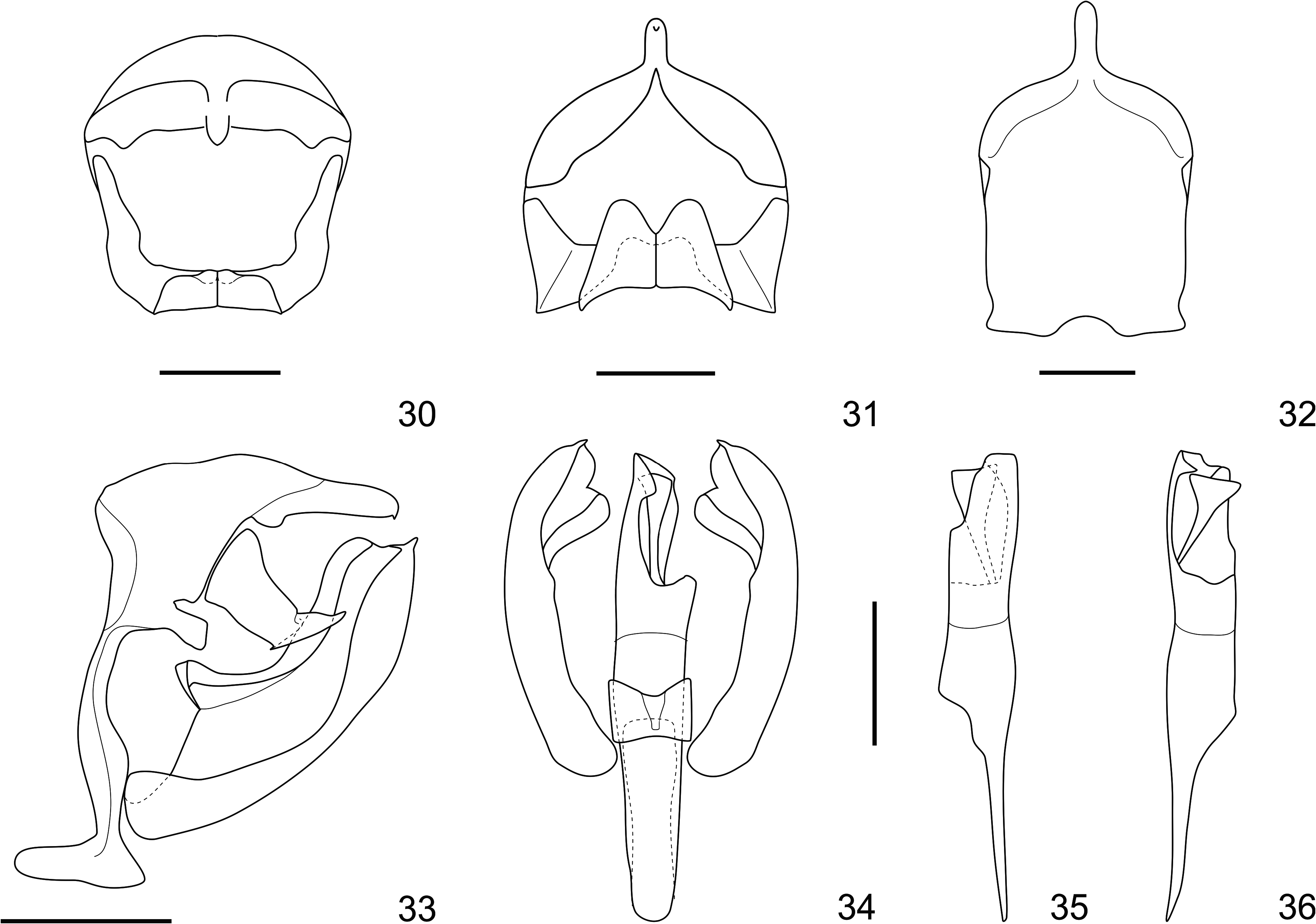
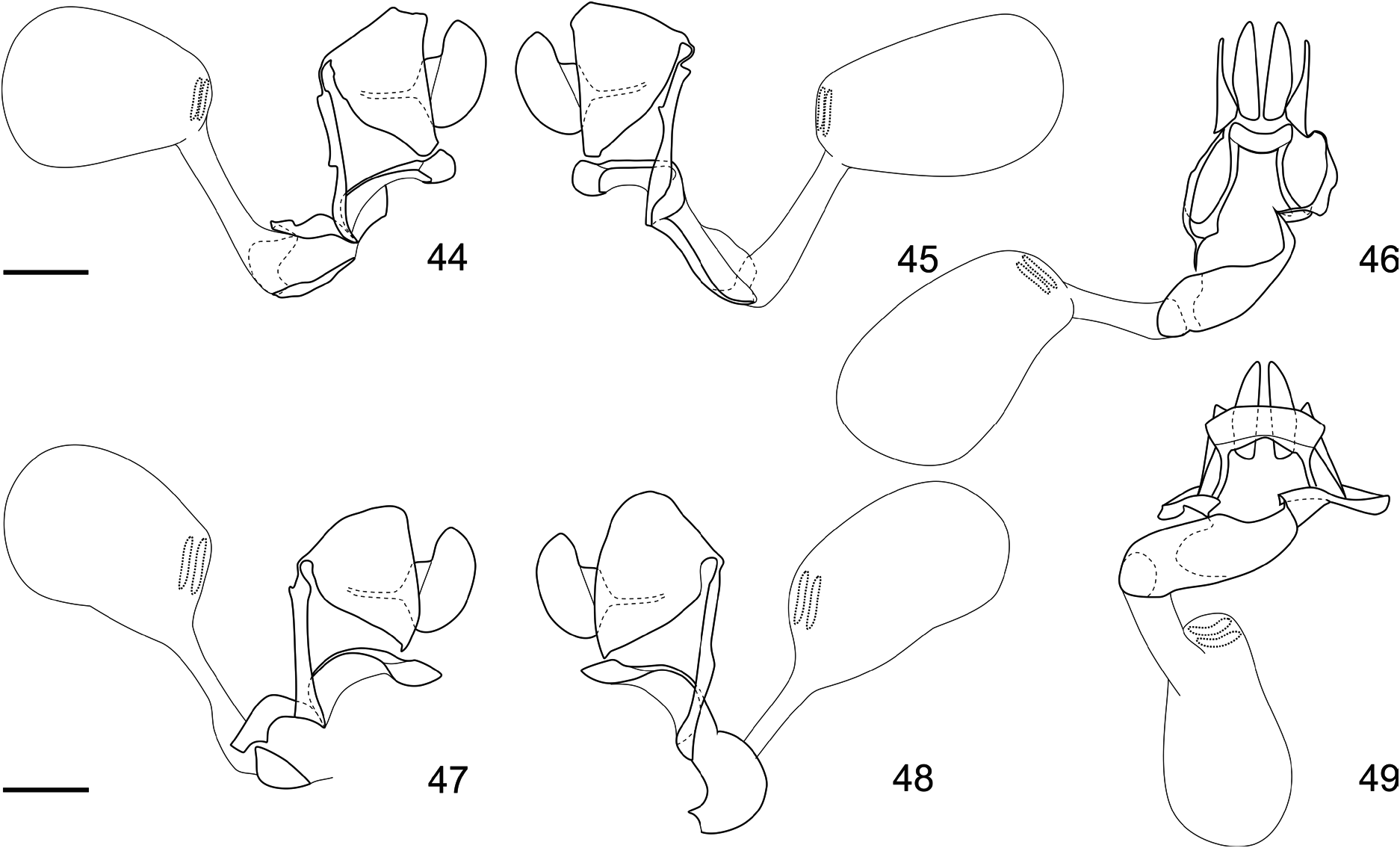
( Figs 1–4 View FIGURES 1–4 , 9 View FIGURE 9 , 11–16 View FIGURES 11–14 View FIGURES 15–18 , 19–36 View FIGURES 19–20 View FIGURES 21–25 View FIGURES 26–29 View FIGURES 30–36 , 44–46 View FIGURES 44–49 , 50 View FIGURE 50 )
Type material: Holotype male with the following labels: / HOLOTYPUS / HOLOTYPE Memphis smalli Riley & Dias det. 2021 / Panama: Bocas del Toro Palo Seco Protect [ed]. Forest Vic [inity of]. Fortuna Cabins. 800 M[eters] 8.78132 oN, 82.19094 oW May 1–6, 2019. T [homas]J[.] Riley [leg.] /TJR-Anaeini-db #0,000,283/ ( USNM) Allotype female with the following labels: / ALLOTYPUS / ALLOTYPE Memphis smalli Riley & Dias det. 2021// Panama: Bocas del Toro Palo Seco Protect [ed]. Forest Vic [inity of]. Fortuna Cabins. 800 M[eters] 8.781325 oN, 82.19094 oW May 16–20, 2018. T [homas]J[.] Riley [leg.] /TJR-Anaeini-db #0,000,274/ ( USNM) . Paratypes (4♂ & 7♀): PANAMA: Ngäbe-Buglé, Jirondai, Büri ( Bosque Protector de Palo Seco ), 1♂ , 1–6.VIII.2019 & 1♀, 1–6.V .2019 , DZ 52.835 (barcoded, dissected), DZ 52.834 (dissected), Riley leg. (DZUP); 1♂ & 1♀, 1–6.VIII.2019, MZUEL (E) 00.001 (barcoded), MZUEL(E) 00.002, Riley leg. ( MZUEL); 1♂ , 1–6.VIII.2019, TJR-Anaeini-db #0,000,011, 1♂, 1–6.V.2019, TJR-Anaeini-db #0,000,282, 1♀, 16–20.V.2018, TJR-Anaeini-db #0,000,273, 1♀ 5.IX.2021, Riley leg. ( TJR), 3♀ , 9.VII.2018, 24.X.2020, 8.VII.2021, MacDonald leg. ( MEM) .
Etymology. The species name honors Gordon B. Small, Jr. (1934-1989), in recognition of his significant contributions to the knowledge of Panamanian butterflies. It is also chosen for his influence on the life and career of many lepidopterists, including TJR who was mentored by Gordon while living in Panama in the 1960s. The name is formed as a noun in the genitive case.
Diagnosis. Memphis smalli sp. nov. can be distinguished from most species of Memphis by the presence of the projection in M 3 of the hind wing in both sexes and by the presence of a shallow tornal emargination in the forewing, and distiguished from most species of the “ verticordia ”, “ arginussa ” and “ appias ” species groups, such as M. herbacea , M. pithyusa , M. arginussa , M. neidhoeferi , M. appias (Hübner, [1825]) , and M. xenocles (Westwood 1850) , by the coloration of the wings, specially by the presence of an iridescent blue sheen and scattered light blue scales in marginal and submarginal areas of the forewing upper side, submarginal light blue spots from R 5 to the tornus in the forewing upper side, and marginal spots from the apex to the tornus in the hind wing upper side (see Warren et al. 2016). Memphis smalli sp. nov. can be further distinguished from M. neidhoeferi by the less scalloped outer margins and by the comparatively reduced amount of speckles in the undersides of the wing. Although M. smalli sp. nov. is more closely related to M. herbacea (and both these species to M. pithyusa ) by molecules and morphology (Figs 5–9, 17–18, 37–43, 47–50), the new species can only be confused with M. perenna , the enigmatic M. xenippa ( Hall, 1935) ( Hall 1935) , and the recently described M. paulus ( Costa et al. 2014), but M. smalli sp. nov. can be distinguished from these three species by the presence of a well-developed light blue spot in M 2 –M 3 on the forewing upper side, and the presence of marginal light blue spots forming a continuous band and reaching the tornus (i.e. present in spaces M 3 –CuA 1, CuA 1 –CuA 2 and CuA 2 –2A) in the hind wing upper side. The genitalia of both sexes are characteristic, but most similar to M. herbacea ( Figs 26–36 View FIGURES 26–29 View FIGURES 30–36 , 44–46 View FIGURES 44–49 ).
Description. Males ( Figs 1–2 View FIGURES 1–4 ) forewing average length 30.2 mm (29.5–30.5mm) (n=6). Females ( Figs 3–4 View FIGURES 1–4 ) forewing average length 35.5 mm (34.8–36.5mm) (n=7).
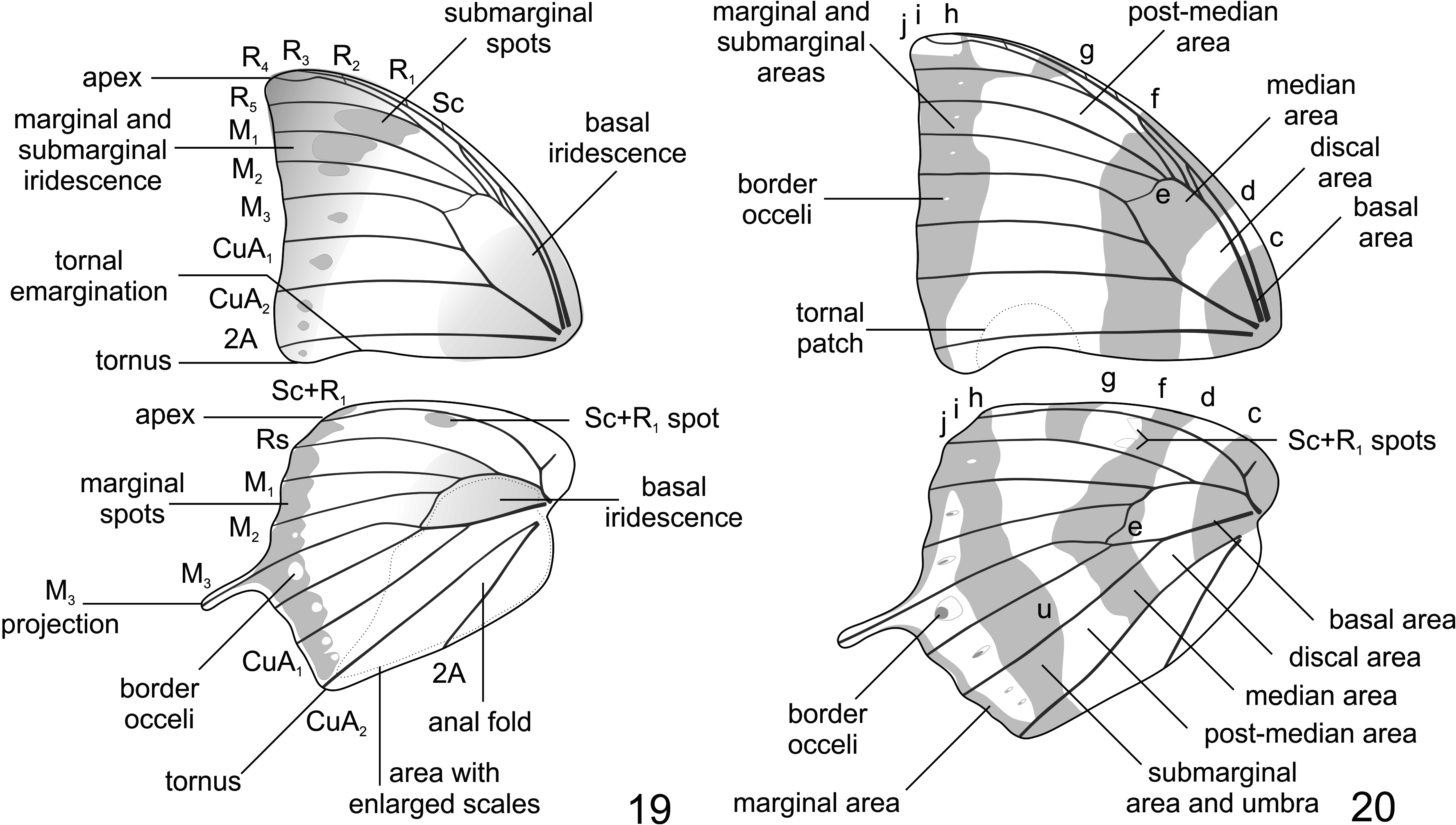
Wing shape ( Figs 1–4 View FIGURES 1–4 , 19–20 View FIGURES 19–20 ): Forewing triangular; costal margin evenly curved; apex pointed; outer margin sinuous, shallower at CuA 1 –CuA 2; tornus rounded; inner margin straight, slightly emarginated before tornus; hindwing humeral area rounded and well-developed; costal margin evenly curved; apex rounded; outer margin slightly curved and slightly crenulated, with a long and blunt projection at vein M 3; tornus right-angled; inner margin slightly curved. Female as in male, but both wings larger; forewing outer margin sinuous but more rounded, shallower at M 1 –M 2; inner margin tornal emargination more developed; hindwing costal margin slightly sinuous; outer margin more rounded and crenulated; tornus obtuse-angled.
Wing color, upper side ( Figs 1, 3 View FIGURES 1–4 , 19 View FIGURES 19–20 ): Ground color of both wings dark brown; wing bases with a variable iridescent greenish blue sheen; veins covered with dark brown scales; forewing with an iridescent greenish blue sheen and scattered light blue scales in marginal and submarginal areas; light blue spots forming submarginal continuous band in spaces R 5 –M 1, M 1 –M 2, M 2 –M 3, the former distinctly larger than the latter two, spot in space M 2 –M 3, smaller and occupying only anterior half of the space; smaller isolated light blue spots in spaces M 3 –CuA 1, CuA 1 –CuA 2, CuA 2 –2A and between 2A and the tornus, two spots in CuA 2 –2A; fringes white; hindwing with a post-median yellowish spot at space Sc+R 1 –Rs; light blue spots forming a marginal continuous band from the apex to the tornus along the outer margin, spots in spaces M 3 –CuA 1, CuA 1 –CuA 2 and CuA 2 –2A not reaching the outer margin; white ocelli in spaces M 2 –M 3, M 3 –CuA 1, CuA 1 –CuA 2 and CuA 2 –2A, the largest in space M 3 –CuA 1; light blue scaling on the base of the M 3 projection; long brown scales in the discal cell and spaces along the inner margin; anal fold developed, with brown scales; fringes white. Female as in male, but forewing with more developed spots and abundant light blue scales scattered in the marginal and submarginal areas; and hindwing post-median yellowish spot at space Sc+R 1 –Rs larger.
Wing color, underside ( Figs 2, 4 View FIGURES 1–4 , 20 View FIGURES 19–20 ): Ground color of both wings with ripple patterns formed by different shades of reddish brown and white scales; forewing with basal and discal area coalesced, mostly light reddish brown with a profusion white scales near the discal band (band d, Nijhout 1991) and the costal margin; median area mostly dark reddish brown with a profusion of white scales near the costal margin; post-median area mostly light reddish brown with a profusion of white scales near the median band (f) and the costal margin, and a distinct semicircular patch bordered by white scales near the inner margin tornal emagination; post-median band (g) proximally displaced near the costal margin; submarginal and marginal areas coalesced, mostly dark reddish brown with a profusion of white scales in the apex and along the outer margin in the submarginal area; minute white ocelli in the submarginal area; fringes white; hindwing with basal and discal area coalesced, mostly light reddish brown with scattered white scales; median area mostly dark reddish brown, from the costal margin to 2A; post-median area mostly light reddish brown, continuous in color with the anal fold area, with two white spots in space Sc+R 1 –Rs near the median band (f); post-median band (g) proximally displaced near the costal margin; submarginal area mostly dark reddish brown with a distinct proximal umbra and a profusion of white scales along the outer margin; black and white ocelli in the submarginal area, more developed in space M 3 –CuA 1, two ocelli in CuA 2 –2A; marginal area dark reddish brown; fringes white. Female color duller, but pattern more discernible than in male.
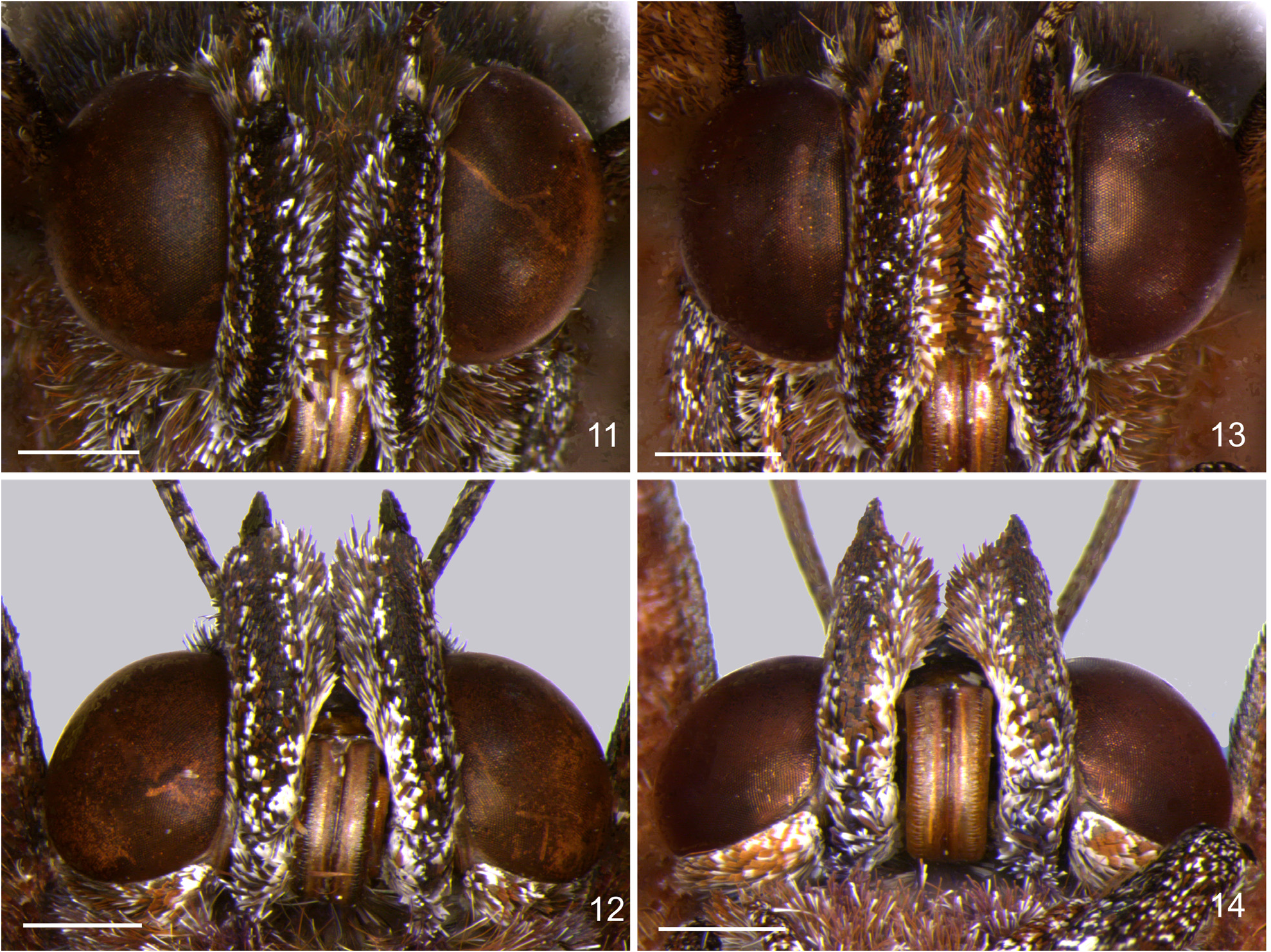
Head ( Figs 11–16 View FIGURES 11–14 View FIGURES 15–18 , 21 View FIGURES 21–25 ): Eyes naked; labial palpi mostly light reddish brown speckled with white scales, with a ventral band formed by dark reddish brown scales; antennal length about half of the forewing length, antennomeres dark brown with speckled ventral white scales; club slender and elongated, tip reddish. Female as in male, but duller in color.
Thorax ( Figs 1–4 View FIGURES 1–4 , 22–25 View FIGURES 21–25 ): Dark brown with iridescent greenish and bluish scaling; ventral color and legs dark reddish brown, with speckled white scales. Female as in male, but duller in color.
Abdomen ( Figs 1–4 View FIGURES 1–4 ): Dorsal surface mostly dark brown, with some iridescent greenish and bluish scaling near the thorax; ventral surface mostly white. Female as in male, but duller in color.
Male genitalia ( Figs 26–36 View FIGURES 26–29 View FIGURES 30–36 ): Tegumen trapezoidal in lateral view, with a lateral lips, fused dorsally to the anterior part of the uncus and laterally to the gnathos, appendix angularis large and rectangular; saccus with anterior and posterior projections, the former twice the size of the latter, dorsal projection slightly curved and attached to the tegumen ventrally; uncus straight, with lateral ridges pointing to a medial tip; gnathos trapezoidal and converging ventrally, fused medially and with projected lobes, posteriorly rounded in ventral view; valva ventrally curved, sacullus smooth, harpe rough, with a posterior pronged projection; ampulla projected and continuous with the costa; costa “C”-shaped, attached to the appendix angularis; aedeagus cylindrical, ductus ejaculatorius aperture large and dorsal; manica attached to the distal third of the aedeagus; aedeagus aperture to the left side, with a slender sclerotized lip; fultura inferior (i.e. juxta of authors) subrectangular, posterior edge bifid.
Female genitalia ( Figs 44–46 View FIGURES 44–49 ): Tergum VIII triangular, attached ventrally to the sides of the lamella postvaginalis, and dorsally to the lamella antevaginalis by a slender loop; lamellae postvaginalis as a transversal folded sclerotization attached to the lamellae antevaginalis by slender sclerotized bands; lamellae antevaginalis slender and assymetrical, left side larger than the right, sinuous and ventrally forming a large semi-cylindrical sclerotization directed to the right side, right side slender and sinuous; ductus bursae inserted at the end of the lamellae antevaginalis ventral sclerotizations; seminal duct inserted close to the base of the ductus bursae; corpus bursae oval, about the length of the ductus bursae, bearing two short, parallel signa immediately dorsal to the ductus bursae insertion, formed by sclerotized bumps; anal papillae oval and bristled, anteriorly projecting a slender posterior apophysis.
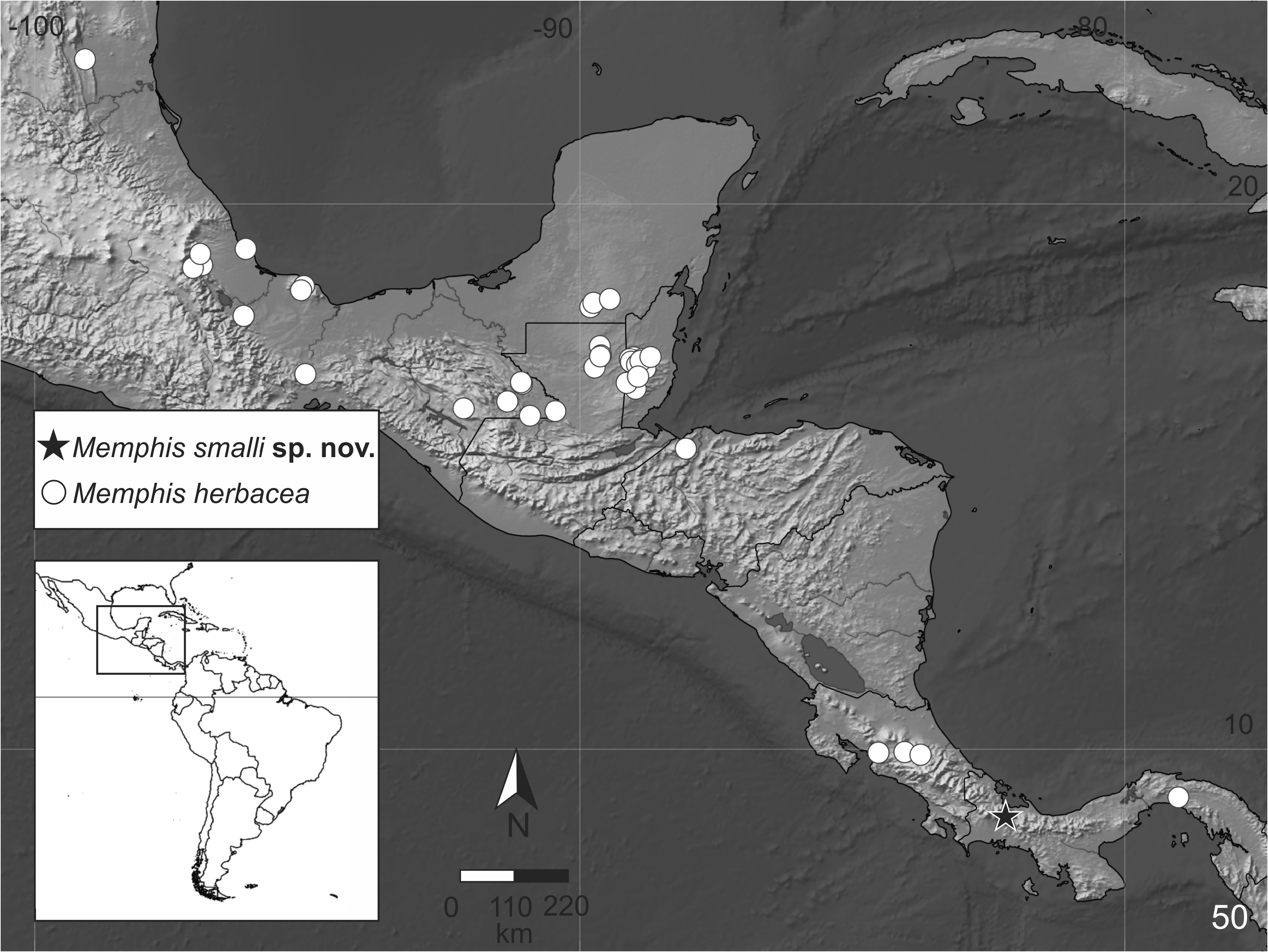
Distribution ( Figs 10 View FIGURE 10 , 50 View FIGURE 50 ). Memphis smalli sp. nov. is known from only one location on the Atlantic slope of Panama, along the paved Gualaca– Chiriqui Grande highway. The vicinity of the type location was surveyed, in both sides of the continental divide and at different elevations, but so far no specimens of M. smalli sp. nov. were collected. The type location ( Fig. 10 View FIGURE 10 ) could be characterized as a mid-elevation (i.e. about 800m) Atlantic rainforest habitat similar to other areas in Panama and Costa Rica, such as the Braulio Carrillo National Park in Costa Rica. This habitat does not appear to be isolated or cut off in other ways from similar habitats on the Atlantic slopes of Panama and Costa Rica. Although mountains may restrict local species movement between valleys or areas of suitable micro-habitat, forests at that altitude extend from the collection site westward and eastward, unbroken all along the Atlantic side of the continental divide in the western half of Panama.
Taxonomic comments. Memphis smalli sp. nov. is described based on thirteen specimens, five males and eight females, collected in the same location in four consecutive years. Three other species of the “ arginussa ” species group are known to occur in the same location: M. neidhoeferi , M. eubaena stat. rest., and M. pithyusa , along with M. artacaena , and M. glauce centralis (Röber, 1916) of the related “ verticordia ” and “ glauce ” species groups, respectively. Nevertheless, M. smalli sp. nov. is easily recognizable from most other central American species of Memphis . It is very distinct in its upper side color and pattern in both the males and females, and the underside color of the females is distinct from most similar females of Memphis . The new taxon not being previously collected may be due to the area having been remote and not easily accessible until recently, and has only been collected using bait traps, similar to M. paulus , described from specimens collected at the Venezuelan “tepuis” ( Costa et al. 2014). As with other species of leafwings, there is a lot of intraspecific variation in wing color in M. smalli sp. nov., verified even in the small number of know specimens. Males of M. smalli sp. nov. are very distinct when flying and seen from above. TJR observed several males fly upward from bait placed on the ground and the light blue hind wing marginal band stands out and makes it immediately recognizable and distinguishable from any of the other Memphis species.
Nevertheless, M. smalli sp. nov. seems to be very closely related to M. herbacea , at least at molecular level. The intrerspecific AED between these two species is 0.5%, well below the 2% AED suggested as an appropriate threshold for species delimitation in Lepidoptera ( Hebert et al. 2003b) . However, there are striking differences between M. smalli sp. nov. and M. herbacea wings in wing shape, color, pattern, and genitalia of both sexes, that should be considered in combination with the molecular data. Most specimens of M. herbacea were collected in lowlands in Mexico (Campeche, Chiapas, Oaxaca, Tamaulipas, and Veracruz states), Belize (Cayo district), Guatemala (Petén department), Honduras (San Pedro Sula, Cortés department), Costa Rica (Alajuela and Cartago provinces) and Panama (Bayano, Panama province). DeVries (1987) cites that “[M.] herbacea occurs from 500–1000 meters on Pacific slope wet-forest habitats and one location [Juan Viñas, Cartago province] on the Atlantic slope”. Although M. herbacea is rather common in the Atlantic slopes of Mexico, Belize, Guatemala and Honduras, the few records available from Costa Rica and Panama are mostly from the Pacific slopes. Therefore, the ranges of M. herbacea and M. smalli sp. nov. probably overlap, at least macro-sympatricaly. It is possible that the contact between the species is prevented because they occupy different ecological niches; for example, while M. herbacea occurs mostly in lower elevations, M. smalli sp. nov. seems to occur exclusively in elevations above 800m. Additionally, if M. smalli sp. nov. should be conspecific with M. herbacea , one would expect to observe a gradual shift in color in the Costa Rican or Panamanian specimens, which were collected not very far from the type location of M. smalli sp. nov. The fact that the color pattern of M. smalli sp. nov. is strikingly different, displaying an abrupt change, in an isolated population, with no traces of intermediate specimens or gradual shift in any M. herbacea specimens support its status as a species and not a subspecies ( Braby et al. 2012), even though the molecular AED is below the suggested threshold for species delimitation using “DNA barcodes”. In fact, similar cases occur in Costa Rica and in mountainous areas of southeastern Brazil, where some macro-sympatric species of skippers and satyrs, respectively, well defined by morphology and habitat, have nearly identical “DNA barcodes” ( Burns et al. 2007; Pyrcz et al. 2018). Cases like these and the one presented herein stress the fact that ideally no single source of data should be used alone for the delimitation of species ( Goulding & Dayrat 2016).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.