Macrobiotus paulinae, Stec, Daniel, Smolak, Radoslav, Kaczmarek, Łukasz & Michalczyk, Łukasz, 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4052.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:901316AF-63D8-4C5A-8F16-D901D5AF5F86 |
|
DOI |
https://doi.org/10.5281/zenodo.6114474 |
|
persistent identifier |
https://treatment.plazi.org/id/062287B7-9F74-FF8A-D597-6579FF664B1F |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus paulinae |
| status |
sp. nov. |
Macrobiotus paulinae View in CoL sp. nov.
( Tables 4–5 View TABLE 4 View TABLE 5 , Figs 1–45 View FIGURES 1 – 3 View FIGURES 4 – 7 View FIGURES 8 – 11 View FIGURES 12 – 15 View FIGURES 16 – 19 View FIGURES 20 – 23 View FIGURES 24 – 29 View FIGURES 30 – 33 View FIGURES 34 – 37 View FIGURES 38 – 41 View FIGURES 42 – 45 )
Material examined. 173 animals and 2 eggs isolated directly from the moss sample and 14 animals and 12 eggs obtained from the in vitro culture. Specimens mounted on microscope slides in Hoyer’s medium, fixed on SEM stubs and processed for DNA sequencing.
Description of the new species.
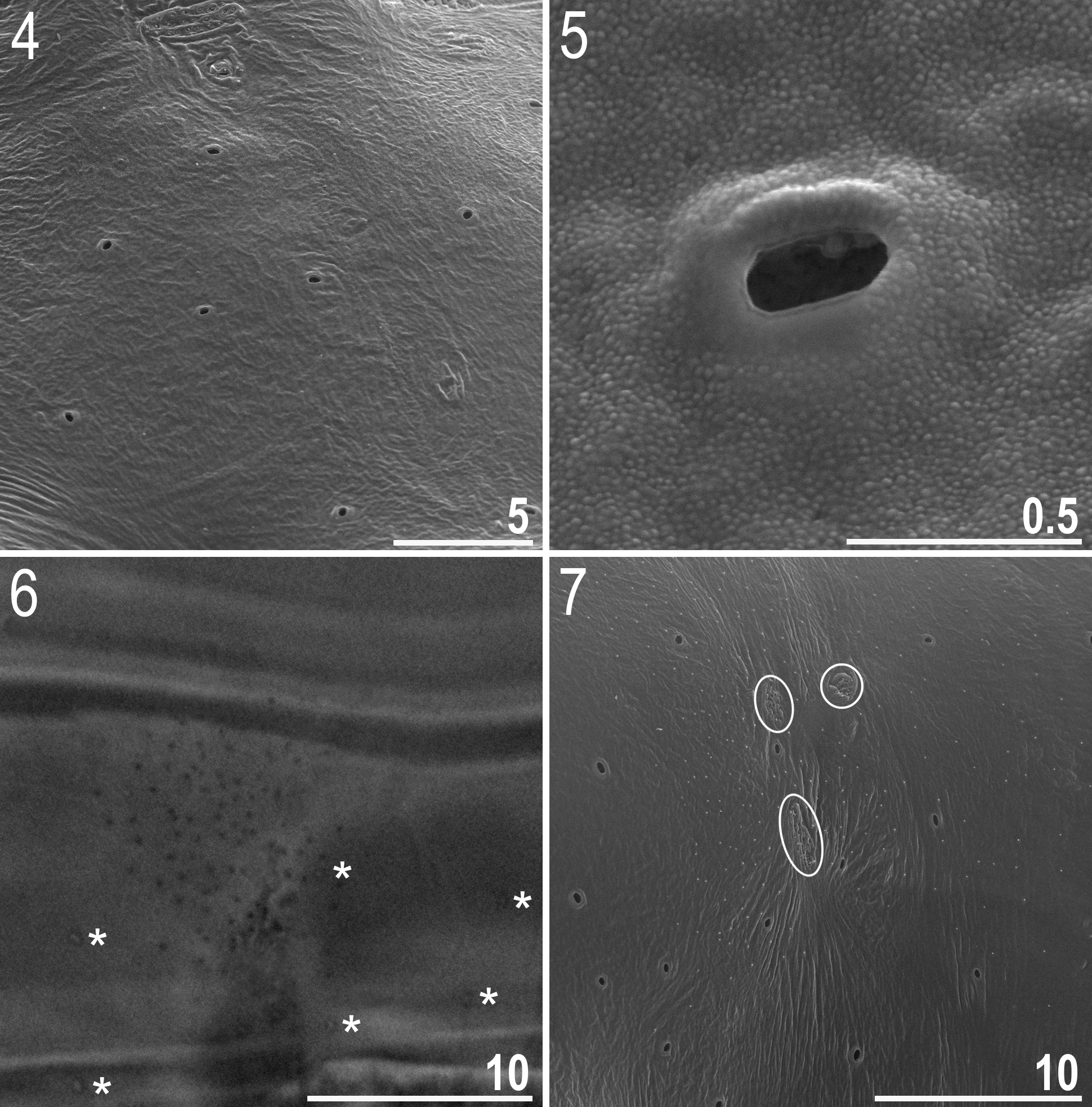
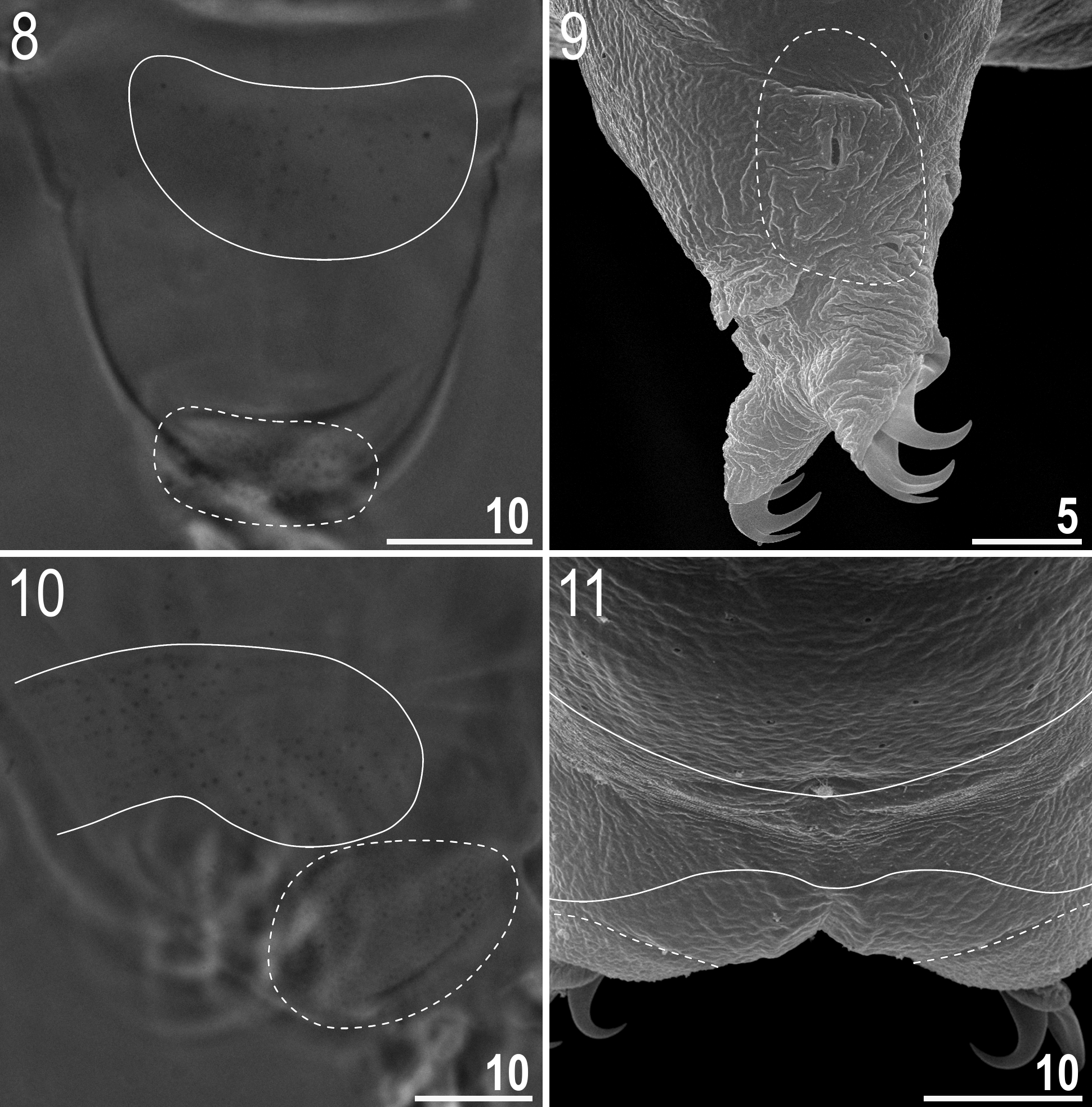
Animals (measurements and statistics in Table 4 View TABLE 4 ): Body from white in juveniles to light yellow in adults, transparent after fixation in Hoyer’s medium ( Fig. 1 View FIGURES 1 – 3 ). In older, live animals the portion of the body posterior to legs III is swollen ( Fig. 2 View FIGURES 1 – 3 ). Eyes present (also in mounted specimens). Cuticle covered with pores and faint granulation ( Figs 3–12 View FIGURES 1 – 3 View FIGURES 4 – 7 View FIGURES 8 – 11 View FIGURES 12 – 15 ). Small oval and round pores, 0.3–0.5 Μm in diameter ( Figs 4–6 View FIGURES 4 – 7 ), are scattered randomly on the entire cuticle, although they are larger on dorso-lateral parts of the body, including the outer surface of legs ( Fig. 9 View FIGURES 8 – 11 ). A ring of pores is present around the mouth opening, below the peribuccal sensory lobes ( Fig. 12 View FIGURES 12 – 15 , empty arrow). Due to the small size of pores, they are difficult to observe under light microscopy (LM) (see Fig. 6 View FIGURES 4 – 7 , asterisks). Granulation is arranged in patches on the dorso-lateral and caudo-dorsal cuticle (body granulation) as well as on the outer surface of all legs (leg granulation) ( Figs 3 View FIGURES 1 – 3 , 6–11 View FIGURES 4 – 7 View FIGURES 8 – 11 ). There are seven dorso-lateral patches of sparse granulation arranged symmetrically on both sides of the body. The patches are spaced regularly, with patches I, III and V being placed slightly anteriorly to legs I, II and III, respectively and with patches II, IV and VI being placed slightly posteriorly to legs I, II and III, respectively. Patch VII is placed between legs III and IV, at the level of the cloaca ( Fig. 3 View FIGURES 1 – 3 ). All body patches are arranged around cribriform areas (circled in Fig. 7 View FIGURES 4 – 7 ). Rarely, and only in some of larger animals, the dorso-lateral patches may extend to the dorsum and join to form seven transverse stripes of granulation, narrow dorsally and wide laterally. The granulation on all legs is arranged into two distinct patches: a small area of fine and dense granulation just above claws (distal patch) and a larger area of more robust and sparse granulation located in the middle of each leg (proximal patch) ( Figs 8–11 View FIGURES 8 – 11 , in all figures the distal patch is outlined with a dashed curve whereas the proximal patch is outlined with a solid curve). Compared to granulation on legs I– III ( Figs 8–9 View FIGURES 8 – 11 ), the granulation on legs IV is more distinct and forms larger patches, with the proximal patches merged into a single transverse band of granulation ( Figs 10–11 View FIGURES 8 – 11 ). Both pores and granulation are poorly visible, with the dorso-lateral patches being the least visible, thus the cuticle has to be observed under a good quality contrast microscope and at the highest magnification in order to ensure correct interpretation.
Mouth antero-ventral ( Fig. 12 View FIGURES 12 – 15 ). Bucco-pharyngeal apparatus of the Macrobiotus type, with the ventral lamina and ten small peribuccal lamellae. The lamellae are only clearly visible under SEM ( Figs 13–14 View FIGURES 12 – 15 ) and very difficult to identify under LM ( Fig. 15 View FIGURES 12 – 15 ). The oral cavity armature composed of three bands of teeth, but under LM only the third band is visible ( Fig. 15 View FIGURES 12 – 15 ) and SEM is required to reveal the first and the second bands of teeth (see Figs 13–14 View FIGURES 12 – 15 ). The first band of teeth comprises extremely small cones arranged in a single row situated at the anterior portion of the oral cavity, on the bases of peribuccal lamellae ( Fig. 13 View FIGURES 12 – 15 , indented arrowhead). The second band of teeth is composed of ca. five rows of slightly larger cones, positioned at the rear of the oral cavity, between the ring fold and the third band of teeth ( Figs 13–14 View FIGURES 12 – 15 , flat arrowheads). The teeth of the third band are positioned at the rear of the oral cavity, between the second band of teeth and the buccal tube opening ( Figs 13–15 View FIGURES 12 – 15 ). Under LM, the teeth of the third band appear as a single ventral and a single dorsal thin transversal ridge ( Fig. 15 View FIGURES 12 – 15 and the lower insert). However, SEM reveals that both ventral and dorsal teeth indeed form continuous ridges, but with evident median and lateral peaks corresponding to median and lateral teeth in species with stronger oral cavity armatures ( Fig. 14 View FIGURES 12 – 15 ). Median teeth are smaller than the lateral teeth, which appears under LM as a thinning in the central portion of the ridges ( Fig. 15 View FIGURES 12 – 15 and the lower insert). In addition, there are a number of smaller accessory teeth placed laterally to the lateral teeth. These accessory teeth are more likely to develop on the ventral side than on the dorsal. Buccal tube walls are slightly thickened posterior to the stylet support insertion point ( Fig. 15 View FIGURES 12 – 15 , empty indented arrowhead). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and a triangular microplacoid ( Fig. 15 View FIGURES 12 – 15 and the upper insert). Macroplacoid length sequence 2<1. First macroplacoid with a central constriction ( Fig. 15 View FIGURES 12 – 15 , upper insert).
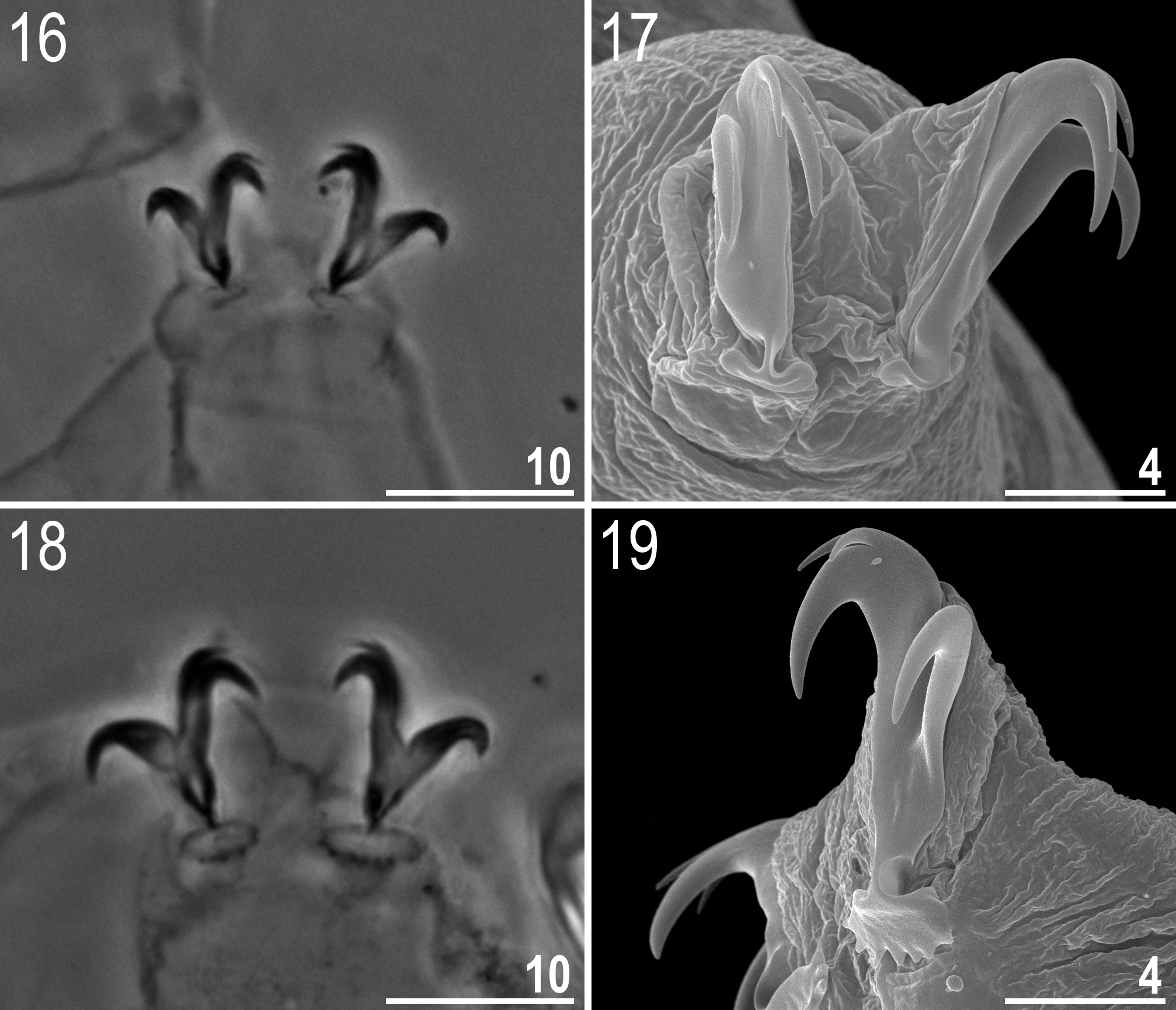
Claws small and slender, of the hufelandi type ( Figs 16–19 View FIGURES 16 – 19 ). Primary branches with distinct accessory points. Lunules on legs I–III smooth ( Figs 16–17 View FIGURES 16 – 19 ), but those on legs IV from crenulate in young animal to dentate in larger specimens ( Figs 18–19 View FIGURES 16 – 19 ). Bars under claws absent, but extremely faint (barely visible under LM, only clearly visible in SEM) paired muscle attachments below claws I–III present.
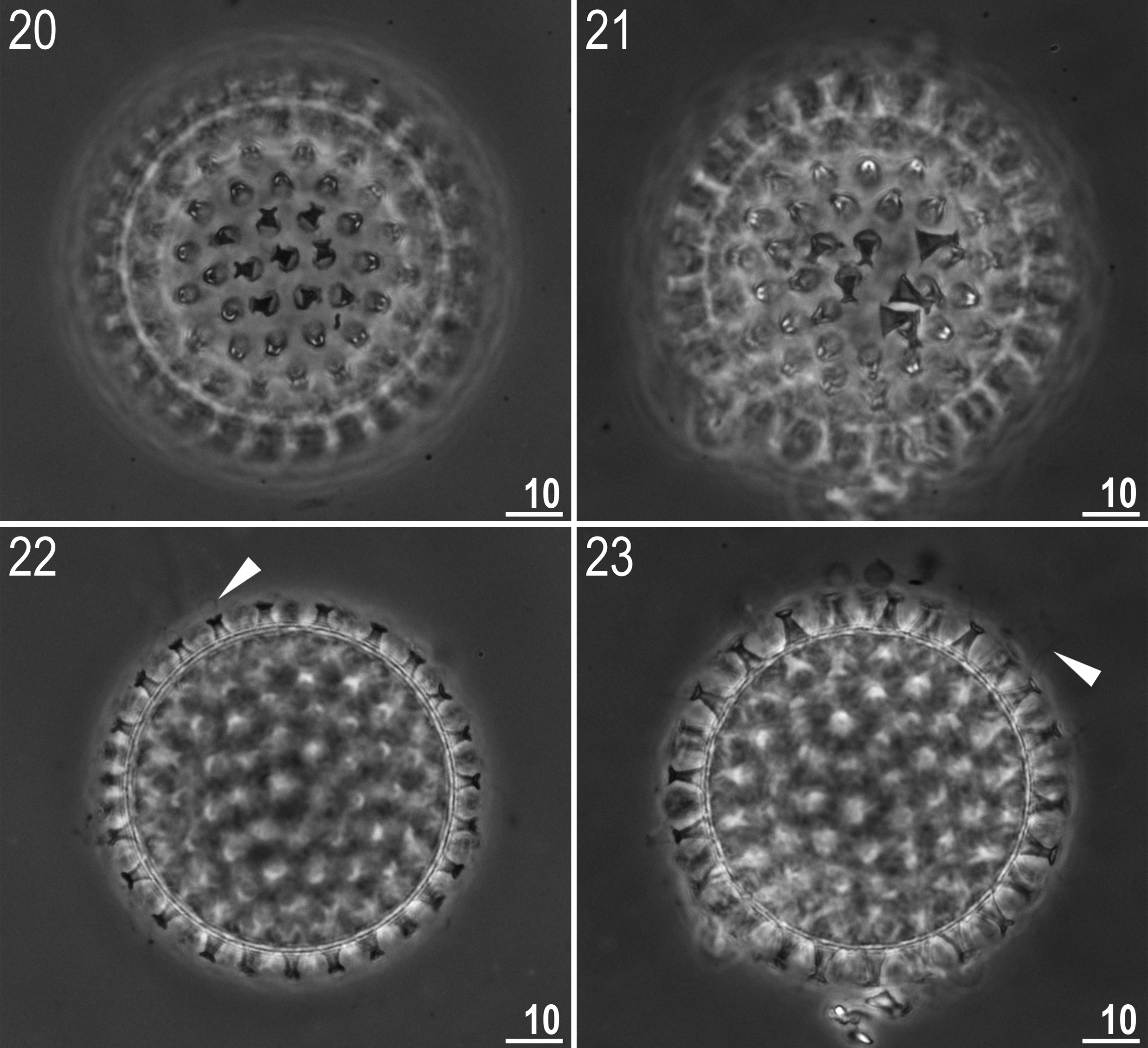
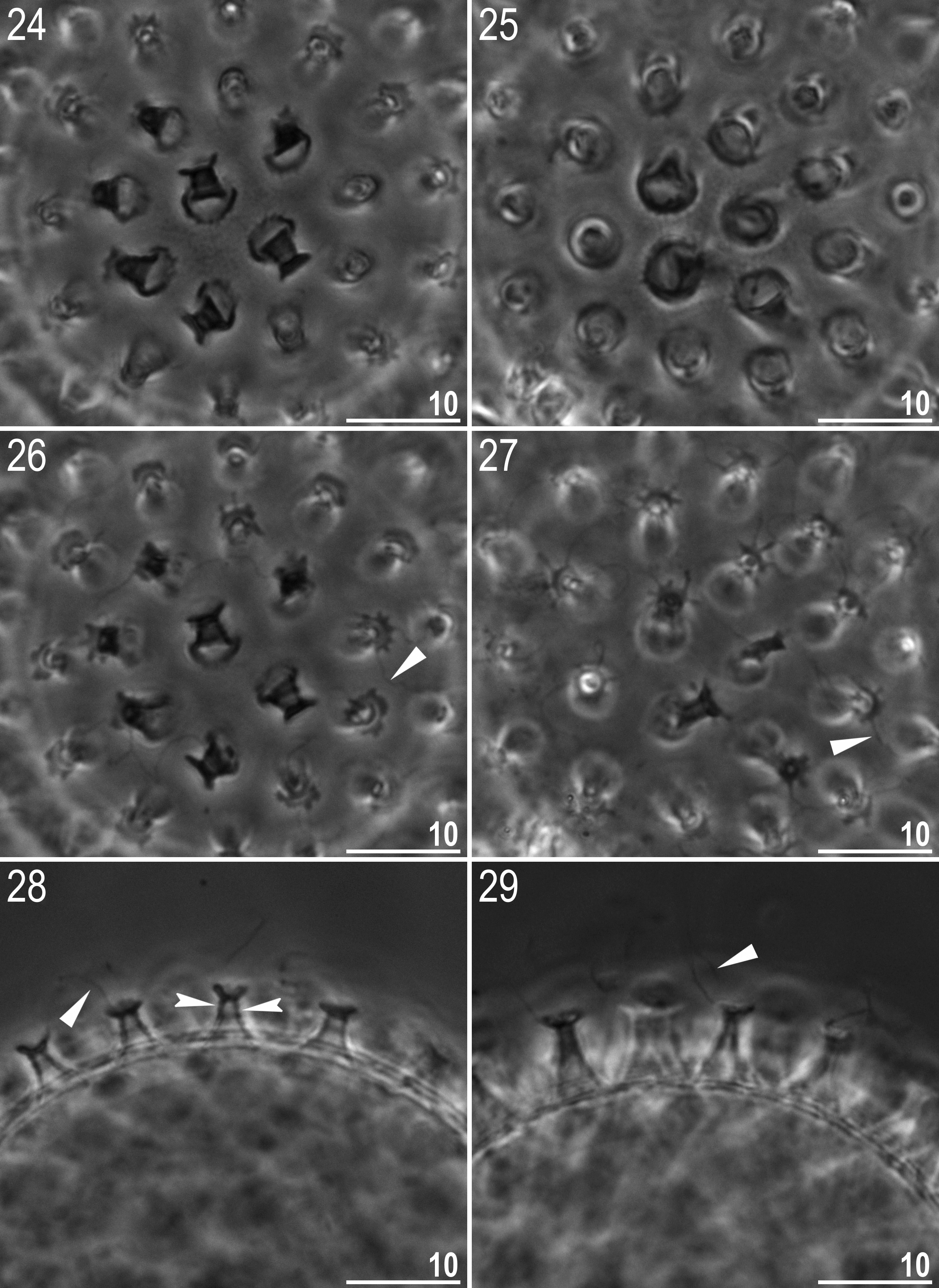
Eggs (measurements and statistics in Table 5 View TABLE 5 ): Laid freely, light yellow, spherical and with a hufelandi type chorion ornamentation ( Figs 20–45 View FIGURES 20 – 23 View FIGURES 24 – 29 View FIGURES 30 – 33 View FIGURES 34 – 37 View FIGURES 38 – 41 View FIGURES 42 – 45 ). The surface between processes is covered with a very dense regular reticulum (mesh diameter 0.05–0.20 Μm, reticulum thickness 0.05–0.30 Μm) ( Figs 32–34 View FIGURES 30 – 33 View FIGURES 34 – 37 , 38, 40–42 View FIGURES 38 – 41 View FIGURES 42 – 45 ). As these diameters are on the margin or below the resolution of LM, the mesh is only clearly visible with SEM. Under LM the mesh outline can merely be detected only on some eggs (usually only around the processes, where meshes are slightly larger), but on the majority of eggs the surface appears to be covered with faint dots when observed with PCM ( Figs 24 and 26 View FIGURES 24 – 29 ). Thus, eggs have to be observed under a good quality contrast microscope and at the highest magnification in order to ensure correct identification. Importantly, however, SEM also does not guarantee the correct identification regarding the chorion sculpture. In some of the eggs we observed with SEM, the reticulum was partially covered by a layer of an unknown substance that fills the mesh and makes the egg surface appear smooth ( Figs 31, 33 View FIGURES 30 – 33 , 35 View FIGURES 34 – 37 , 39, 41 View FIGURES 38 – 41 and 43 View FIGURES 42 – 45 ). Figures 33 View FIGURES 30 – 33 and 41 View FIGURES 38 – 41 show a transition from a clearly visible reticulum to an obscured smooth surface. As eggs with an obscured reticulum appear smooth, they could be erroneously classified as the persimilis type ( i.e. with a smooth chorion). Thus, the examination of a larger number of eggs and at magnifications>30,000× is necessary in order to avoid false interpretations.
Processes are in the shape of inverted goblets with slightly concave conical trunks and well-defined terminal discs ( Figs 20–45 View FIGURES 20 – 23 View FIGURES 24 – 29 View FIGURES 30 – 33 View FIGURES 34 – 37 View FIGURES 38 – 41 View FIGURES 42 – 45 ). When observed under SEM, all trunks have 5–7 distinct ring undulations ( Figs 36–41 View FIGURES 34 – 37 View FIGURES 38 – 41 ), whereas in LM the undulations are lightly outlined only in some processes ( Fig. 28 View FIGURES 24 – 29 , indented arrowheads), with the majority of processes appearing to have smooth trunks. Processes exhibit a considerable variation in height and, therefore, also in the base/height ratio ( e.g. see Figs 22–23 View FIGURES 20 – 23 ). Terminal discs are cog-shaped, with a concave central area and with 8–10 small irregular teeth ( Figs 28–29 View FIGURES 24 – 29 , 34–45 View FIGURES 34 – 37 View FIGURES 38 – 41 View FIGURES 42 – 45 ). In SEM, small granules 0.05–0.08 Μm in diameter, are visible on the teeth ( Figs 42–45 View FIGURES 42 – 45 ). Approximately half the processes have one to a few teeth on the disc that are elongated into thin flexible filaments, less than 0.5 Μm in diameter and up to 20 Μm in length ( Figs 34–41 View FIGURES 34 – 37 View FIGURES 38 – 41 ). The filaments are hair-like under LM, but under SEM they are covered with the same type of granulation as the disc teeth ( Fig. 41 View FIGURES 38 – 41 ), so they probably enhance the adhesive function of egg processes. Eggs found in the sample were devoid of filaments and we were able to observe them only on eggs obtained from cultured animals. The most probable explanation for this is that the filaments are very fragile and easily broken, thus are not present on some eggs. Also, the filaments are very thin so could be overlooked and/or misinterpreted as dirt attached to eggs. Therefore, extreme care must be taken when examining the eggs in order to avoid incorrect conclusions.
DNA sequences. First, we sequenced all four DNA fragments for four individuals. As we found no polymorphism in any of the sequences, for the remaining ten individuals we have amplified and sequenced only ITS-2, potentially the most variable fragment. Again, there were no differences between all fourteen sequences. Since the type population exhibited only one haplotype, only a single sequence for each of the four DNA fragments was uploaded to the GenBank. The typical DNA sequences for Ma. paulinae sp. nov. are as follows:
The 18S rRNA sequence (GenBank: KT935502 View Materials ), 1731 bp long:
AGATTAGCCATGCATGTCTCAGTACTTGCTTTTACAAGGCGAAACCGCGAATGGCTCATTAAATCAGTTATGGTTCACTA GATCGTAAATTTTACACGGATAACTGTGGTAATTCTAGAGCTAATACGTGCAAGCAGCTCGTTTCCTTGTGGAGCGAGCG CAGTTATTAGAACAAGACCAATCCGGCCTTCGGGTCGGTACAATTGGTGACTCTGAATAACCGAAGCGGAGCGCATGGTC TCGTACCGGCGCCAGATCTTTCAAGTGTCTGACTTATCAGCTTGTTGTTAGGTTACGTTCCTAACAAGGCTTCGACGGGT AACGGGGTATTAGGGTCCGATACCGGAGAGGGAGCCTGAGAAACGGCTACCACATCCAAGGAAGGCAGCAGGCGCGCAAA TTACCCACTCCTAGCACAGGGAGGTAGTGACGAAAAATAACGATGCGAGGGCTAATAGCTTCTCGTAATCGGAATGGGTA CACTTTAAATCCTTTAACGAGGATCTATTGGAGGGCAAGTCTGGTGCCAGCAGCCGCGGTAATTCCAGCTCCAATAGCGT ATATTAAAGTTGCTGCGGTTAAAAAGCTCGTAGTTGGATCTGGGCTTCTGAATGGACGGTTCACTTTACGGTGTAACTGC TCGTTTGGTGCCACAAGCCGGCCATGTCTTGCATGCCCTTTGCTGGGTGTGCTTGGCGACCGGAACGTTTACTTTGAAAA AATTAGAGTGCTCAAAGCAGGCGTATGGCCTTGCATAATGGTGCATGGAATAATGGAATAGGACCTCGGTTCTATTTTGT TGGTTTTCGGAACTCGAGGTAATGATTAAGAGGAACAGACGGGGGCATTCGTATTGCGGCGTTAGAGGTGAAATTCTTGG ATCGTCGCAAGACGAACTACTGCGAAAGCATTTGCCAAGAATGTTTTCATTAATCAAGAACGAAAGTTAGAGGTTCGAAG GCGATCAGATACCGCCCTAGTTCTAACCATAAACGATGCCAACCAGCGATCCGTCGGTGTTTTTTTTATGACTCGACGGG CAGCTTTCCGGGAAACCAAAGTGCTTAGGTTCCGGGGGAAGTATGGTTGCAAAGCTGAAACTTAAAGGAATTGACGGAAG GGCACCACCAGGCGTGGAGCCTGCGGCTTAATTTGACTCAACACGGGAAAACTTACCCGGCCCGGACACTGTAAGGATTG ACAGATTGAGAGCTCTTTCTTGATTCGGTGGGTGGTGGTGCATGGCCGTTCTTAGTTGGTGGAGCGATTTGTCTGGTTAA TTCCGATAACGAACGAGACTCTAGCCTGCTAAATAGCCGACCGATCCGCAGCGTCGGTTGCTACAAAAGCTTCTTAGAGG GACAGGCGGCGTTTAGTCGCACGAGATTGAGCAATAACAGGTCTGTGATGCCCTTAGATGTCCGGGGCCGCACGCGCGCT ACACTGAAGGGACCAGAGTGCTTAACTACCTTGGCCGGAAGGCCTGGGGAATCCGGTTAAACCCCTTCGTGATTGGGATT GAGCTTTGTAATTATCGCTCATGAACGAGGAATGCCCAGTACTCGCGAGTCATAAGCTCGCGATGATTACGTCCCTGCCC TTTGTACACACCGCCCGTCGCTACTACCGATTGAATGATTTAGTGAGGTCTTCGGACTGGCCGTCGATGCTGACTCTGTT GGCGTCGGTTGGATCGGAAAGACGACCAAACTGGCTCATTAGAGGAAGTAA
The 28S rRNA sequence (GenBank: KT935501 View Materials ), 788 bp long:
TACTAAGCGGAGGAAAAGAAACCAACGGGGATGCCGATAGTACTGCGAGTGAAATCGGCCAAGCCCAGCGCCGAATCCTG TTGCTGGCGACGGTGACAGGAACTGTGGCGTGAAGAACGTCCTTACCGGTACGGTTTGCGTGCGTAAGTTCTCCTGAGTG AGGCTCCATTCCAAGGAGGGTGCAAGACCCGTATCGCGTGCAACCGGTGTCGGTGTAAGATGTTCGGAGAGTCGCCTTGT TTGTGAGTACAAGGTGAAGTCGGTGGTAAACTCCATCGAAGGCTAAATATGACCACGAGTCCGATAGCGAACAAGTACCG TGAGGGAAAATTGAAAAGCACTTTGAAGAGAGAGCGAAACAGTGCGTGAAACCGCTCAGAGGCAAGCAAATGGGGCCTCG AAGGCAAGGCAGCGAATTCAGCTGGTGGTCTGCGCGGCTGGTTGGTTTGGAGATCTTACGACTCTGGCCGGCTGGGCTCT GAGCGTAGGTGCCAGTGCACTTTTGTTGCTTGTACGCCACCGCCGTTGAGTGGGCATCCGTCGAGTTGGCAATGCGAAGC CTTGAGCCTTTACGGGCCTAGGTGCTTGCAGCCGGCTTTTGTACGCGTTTGCACTTCAACCGGTCATGTTTGCATGTGCC AGCAATTTGGCGTTGGATCGGCTTGCTCTGCCGTTTGTCGTGAGATGACGAGCTTGCTCGGCTCTTCGGCATCTATGGTA GAATCGGGTCGGTTTCAACGTGGGCACATTGTAATTCGGTGGCGAGTAGATGGCTGCCCATTTAACCC
The COI sequence (GenBank: KT951668 View Materials ), 707 bp long:
TTGACAGCTTGTGTAGGAACATCACTAAGCTTTTTAATCCGAACAGAACTCAGCCWACCAGGACTTCTTTTAGCTGATGA ACAAATATATAATGTCATTGTCACAAGCCACGCCTTTATTATAATTTTTTTTTTTGTAATGCCAATTCTTATTGGAGGAT TTGGTAATTGATTAATCCCTTTAATAATCAGAGCCCCCGACATAGCTTTCCCTCGTATAAATAATTTAAGATTTTGAATA CTACCTCCCTCATTTCTTCTAATTACATTAAGAACTATGGCTGAACAGGGAGCCGGTACGGGATGAACTGTGTACCCCCC TCTCTCGCATTTTTTTGCTCATAGGGGGCCTAGGGTAGACTTGACTATTTTTTCTCTTCATGTAGCCGGTATCTCCTCCA TTTTAGGGGCTATTAATTTTATTTCTACAATTATTAATATGCGAGCCCCGTTTATAAGGTTAGAAAAAATACCTCTTTTT GTTTGGTCAGTACTCCTAACTGCCATTTTACTTTTATTAGCTCTGCCTGTGCTTGCAGGGGGCATCACTATATTATTACT AGACCGAAATTTTAACACTTCTTTTTTTGACCCTGCGGGCGGGGGAGACCCTATTTTATACCAACATTTATTTTGATTTT TTGGGCATCCCGAAGTGTATATTTTAATTTTGCCTGGTTTTGGGATTATCTCTCAAATTGTAATTCA
The ITS-2 sequence (GenBank: KT935500 View Materials ), 440 bp long:
AAAATGCGAGACGTAACGTGAATTGCAGGACTTTGTGAACGTTAATTCTTCGAACGCACATTGCGGCTTCGGGTTAACTG AAGCCATGCCTGGTTGAGGGTCAGTTGAAATAAAAAATCGTAATCGYGCATTGATTACGGATTGTCTGGTTTTAACGGCC TTGTGTGCCGTTTCCGGATAAAGTTGAGACCAGATGTGTGCGCTCATTTGACCGGTGCAAGCAACGCTTTGCCGAGTTGG AGCATCCGACTTGTTTAGTCGTGCGCCGCAGCTGCACAATGGCTAAGCATGGTCAACCAACGGCGTTTGATGGCAAAGAA AGACTGGTACAAAAGTGCGCAAGCGCATAGACACGTCTGTGGCCGAAAAAGAACGCACCCAAGTGTGTTTTTGCTCATTC TTTTGACCTCAGCTCAGACAAGATTACCCGCTGAACTTAA
Type locality. 02°39'15.75''N, 36°56'9.99''E; 1824 m asl: Kenya, Eastern Province, Marsabit County, Mount Kulal Biosphere Reserve, Kulal Mt., near Gatab. Habitat: compact high, dense and shady forest (with approximately 30 min of sunshine a day reaching the forest floor). Sample: moss from a dead fallen tree ( Chionanthus sp.). Coll. Radoslav Smolak.
Etymology. The species is named after a former member of the Michalczyk lab and the first author’s friend, Miss Paulina Kosztyła , who wears braids that resemble fine filaments on the egg processes of the new species.
Type depositories. Holotype: slide KE.001.01 (with 17 paratypes), 108 paratypes (slides: KE.001/*, where the asterisk can be substituted by any of the following numbers: 1, 2, 3, 4, 5, 6, 8, 9, 11, 13, 14, 16, 17, 18, 19, 22, 26, 27, 28) and 7 eggs (slides: KE.001/*: 21, 23, 24) are deposited at the Department of Entomology, Institute of Zoology, Jagiellonian University, Gronostajowa 9, 30-387, Kraków, Poland; 25 paratypes (slides: KE.001/*: 10, 12, 15, 20) and 4 eggs (slide KE.001/25) are deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University in Poznań, Umultowska 89, 61-614 Poznań, Poland.
TABLE 4. Measurements [in µm] of selected morphological structures of individuals of Macrobiotus paulinae sp. nov. mounted in Hoyer’s medium (N—number of specimens / structures measured, RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | Holotype |
|---|---|---|---|---|---|
| µm pt | µm pt | µm pt | µm pt | ||
| Body length | 15 | 237–400 837–1165 | 310 1000 | 46 98 | 262 923 |
| Buccal tube | |||||
| Length | 15 | 28.3–35.6 – | 30.9 – | 2.2 – | 28.4 – |
| Stylet support insertion point | 15 | 19.9–26.7 69.7–75.0 | 22.3 71.9 | 1.9 1.7 | 20.7 72.9 |
| External width | 15 | 2.5–4.5 8.8–12.6 | 3.2 10.4 | 0.5 1.1 | 2.8 9.9 |
| Internal width | 15 | 1.0–2.9 3.5–8.1 | 1.7 5.6 | 0.5 1.3 | 1.6 5.6 |
| Ventral lamina length | 15 | 15.0–19.6 51.1–58.3 | 17.0 54.9 | 1.4 2.0 | 15.2 53.5 |
| Placoid lengths | |||||
| Macroplacoid 1 | 15 | 4.6–8.6 16.1–24.2 | 6.6 21.2 | 1.0 2.0 | 6.0 21.1 |
| Macroplacoid 2 | 15 | 3.4–5.8 11.6–16.3 | 4.1 13.1 | 0.6 1.2 | 3.7 13.0 |
| Microplacoid | 15 | 1.3–2.4 4.5–7.7 | 1.9 6.3 | 0.3 0.9 | 2.0 7.0 |
| Macroplacoid row | 15 | 9.2–14.4 32.5–40.4 | 11.4 36.9 | 1.4 2.5 | 10.1 35.6 |
| Placoid row | 15 | 10.8–17.4 37.8–48.9 | 13.7 44.0 | 1.8 3.2 | 12.1 42.6 |
| Claw 1 heights | |||||
| External primary branch | 15 | 8.4–12.4 28.9–38.6 | 9.9 31.8 | 1.2 2.4 | 9.5 33.5 |
| External secondary branch | 15 | 6.9–10.1 23.0–31.5 | 8.2 26.4 | 1.0 2.3 | 8.2 28.9 |
| Internal primary branch | 15 | 8.2–10.9 25.9–32.4 | 9.1 29.4 | 0.8 1.7 | 8.9 31.3 |
| Internal secondary branch | 14 | 6.1–8.6 21.6–26.5 | 7.5 24.2 | 0.7 1.5 | 7.3 25.7 |
| Claw 2 heights | |||||
| External primary branch | 14 | 8.6–12.4 30.1–38.6 | 10.2 33.1 | 1.2 2.7 | 9.6 33.8 |
| External secondary branch | 14 | 7.0–9.3 24.5–29.2 | 8.3 27.1 | 0.6 1.3 | 8.2 28.9 |
| Internal primary branch | 15 | 8.0–11.4 27.4–32.9 | 9.4 30.4 | 0.9 1.6 | 9.2 32.4 |
| Internal secondary branch | 15 | 6.9–8.6 21.8–27.9 | 7.7 24.9 | 0.6 1.7 | 7.9 27.8 |
| Claw 3 heights | |||||
| External primary branch | 12 | 8.8–13.5 31.0–42.1 | 10.9 34.8 | 1.6 3.2 | 8.8 31.0 |
| External secondary branch | 12 | 7.1–11.5 24.5–35.8 | 8.6 27.5 | 1.2 3.1 | 7.7 27.1 |
| Internal primary branch | 11 | 8.3–11.3 28.3–35.2 | 9.4 30.7 | 0.9 1.8 | 8.6 30.3 |
| Internal secondary branch | 10 | 6.7–8.6 21.8–26.8 | 7.7 25.1 | 0.7 1.6 | 6.9 24.3 |
| Claw 4 heights | |||||
| Anterior primary branch | 14 | 8.8–14.7 31.1–45.8 | 11.1 35.7 | 1.8 3.9 | 10.2 35.9 |
| Anterior secondary branch | 14 | 7.4–12.5 24.3–35.8 | 8.8 28.4 | 1.6 3.7 | 9.2 32.4 |
| Posterior primary branch | 14 | 10.0–16.8 35.0–47.2 | 12.2 39.3 | 1.9 3.7 | 11.8 41.5 |
| Posterior secondary branch | 13 | 7.9–12.8 27.6–39.9 | 9.9 31.7 | 1.4 3.3 | 9.7 34.2 |
TABLE 5. Measurements [in µm] of selected morphological structures of eggs of Macrobiotus paulinae sp. nov. mounted in Hoyer’s medium (N—number of eggs / structures measured, RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD |
|---|---|---|---|---|
| Diameter of egg without processes | 11 | 57.0–70.5 | 62.7 | 4.9 |
| Diameter of egg with processes | 11 | 66.3–85.6 | 74.1 | 6.2 |
| Process height | 33 | 3.8–8.1 | 5.6 | 1.0 |
| Process base width | 33 | 3.6–7.7 | 5.0 | 1.0 |
| Process base/height ratio | 33 | 58%–134% | 92% | 18% |
| Terminal disc width | 33 | 2.3–4.2 | 3.4 | 0.5 |
| Distance between processes | 33 | 1.4–5.0 | 2.8 | 0.8 |
| Number of processes on the egg circumference | 11 | 24–32 | 27.6 | 2.4 |
| DNA |
Department of Natural Resources, Environment, The Arts and Sport |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |