Kalanchoe × houghtonii Ward (2006: 94)
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.524.4.2 |
|
DOI |
https://doi.org/10.5281/zenodo.5684317 |
|
persistent identifier |
https://treatment.plazi.org/id/092F87C1-064F-DB0F-FF28-CFDA2E8AF84A |
|
treatment provided by |
Plazi |
|
scientific name |
Kalanchoe × houghtonii Ward (2006: 94) |
| status |
|
1. Kalanchoe × houghtonii Ward (2006: 94)
Homotypic synonym:— Bryophyllum × houghtonii (D. B. Ward) Forster (2006: 383) . Type :— U.S.A., Florida, central Merritt Island , Brevard County, 10 February 2000, D.B. Ward 10700 [collected with R. B. Huck] (holotype, consisting of three parts, FLAS!; isotypes FTG, NY!, SD, US!, USF) View Materials .
Designations not validly published:—‘ Bryophyllum tubimontanum ’ Houghton (1935: 44) ; “ Kalanchoe × hybrida HORT. View in CoL ”
Taxonomic notes:—The first documented synthesis of material today known as K. × houghtonii (= K.daigremontiana × K. tubiflora ) was when Arthur Duvernoix Houghton (1870–1938) recorded the hybrid as having been produced in the USA ( Houghton 1935: 44). By 1954, i.e., within 20 years, material with the parentage K. daigremontiana and K. tubiflora was featured and illustrated in the first German edition of Jacobsen (1986: 615, Fig. 860) as “ Kalanchoe × hybrida HORT. ”, and also treated (but not illustrated) as “ K. cv. Hybrida” by Jacobsen (1977: 285). Whether the material that Jacobsen had access to originated from the laboratory of Resende, who created the hybrid in Lisbon, Portugal ( Resende 1956: 241–242), or from Houghton in the USA, or from elsewhere, is not known.
Material that was eventually described as K. × houghtonii has become successfully naturalised in Florida, USA, although it is not known whether the material so recorded originated from beyond Florida and was carried there, or whether the hybrid material arose in the state where the putative parents were growing socially ( Ward 2006: 94). Kalanchoe × houghtonii has also become established and sometimes weedy in mild-climate regions on six continents (Smith 2019, Herrando-Moraira et al. 2020). We therefore deliberately clarify and elaborate on previous taxonomic treatments of this nothospecies (see for example Shaw 2008). In addition, some confusion has existed regarding the correct application of the name K. × houghtonii, with, for example, Maire (1976: 266–267) and Sajeva & Costanzo (2000: 172, top left image) illustrating and describing material of this nothospecies under the name K. daigremontiana .
Overall, we recognise four morphotypes in what are likely K. × houghtonii, two of which are of known synthetic origin, and another two seem to occur naturally and may represent evidence of gene flow and introgression between K. daigremontiana and K. tubiflora where they grow sympatrically in their natural habitat in Madagascar.
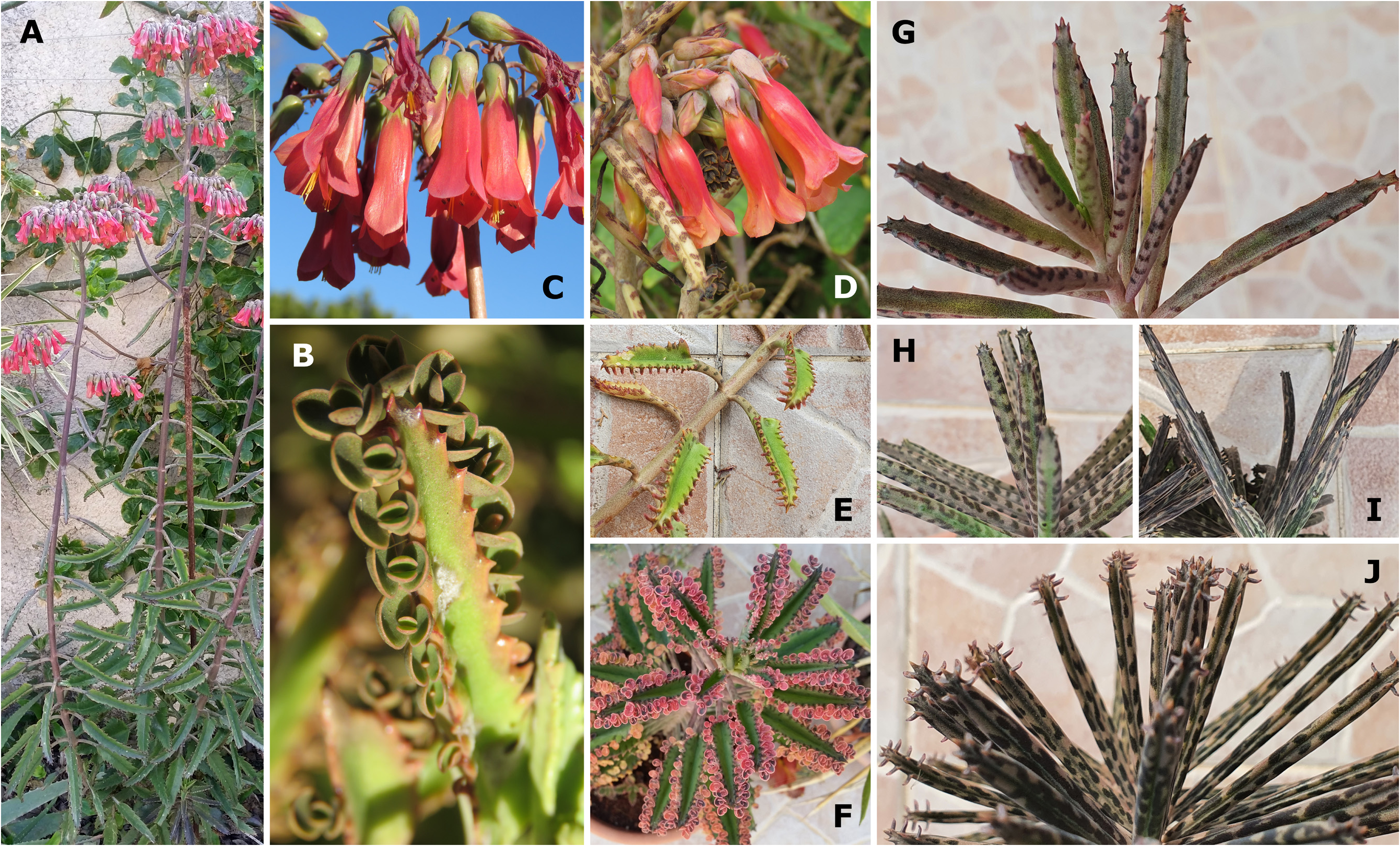
A. Kalanchoe × houghtonii Morphotype A ( Fig. 1A–C View FIGURE 1 )
Plants of Morphotype A have triangular-lanceolate leaf blades that are attenuate (when young) to subpeltate (when mature) basally, and large, inflated flowers reminiscent of those of K. tubiflora . The corolla is usually magenta, red in strong sun, rather than orange, and the leaves are much larger than those of Morphotype B (see [2.] below), so resulting in confusion between Morphotype A and K. daigremontiana (one of the parents of the nothospecies). A further similarity with K. daigremontiana is evidenced in the slower transition in leaf arrangement from decussate to tricussate, with plants remaining decussate for a longer period, before an alternate or tricussate arrangement can be observed (whereas K. tubiflora is tricussate). Leaves of plants of this group are generally only purplish brownmaculate abaxially and along the petiole.
Plants of Morphotype A have been produced artificially more than once on different continents (continental Europe and the USA; see Resende 1956: 241–242 and Ward 2006, 2008), and at least in the USA they seem to be derived from one of two clones originally produced by Houghton, according to Shaw (2008: 22) (cited by Herrando-Moraira et al. 2020). However, Houghton (1935: 44) only presented a single clone of this formula, which, based on the single figure he provided, matches rather K. × houghtonii Morphotype B (see [2.] below). As noted by Shaw (2008: 22), K. × houghtonii was illustrated in Baldwin (1949: 344, Fig. 1 View FIGURE 1 ); however, Baldwin (1949: 343) only reported the chromosome number of a single clone of the nothospecies (presumably the one illustrated), as being 2 n = 51. i.e., a triploid, given that the chromosome numbers earlier reported for K. daigremontiana and K. tubiflora [as K. verticillata Scott Elliot (1891: 14) ] were 2 n = 34 (diploid) and 2 n = 68 (tetraploid), respectively ( Baldwin 1938: 576). Baldwin (1938) did not report a chromosome count for the hybrid for which the name K. × houghtonii was eventually published. Note that some cytological variation was later found in K. daigremontiana , with chromosome numbers of 2 n = 34, 60, 68, 176 reported by Sharma & Gosh (1967: 318–319). However, we were unable to verify the identity of the material investigated; the counts may have been based on artificially raised selections or on forms of the nothospecies, such as K. × houghtonii Morphotype A, which is often confused with K. daigremontiana as stated above.
The plant investigated by Baldwin (1949) likely represents the material recorded by Houghton (1935: 44), i.e., K. × houghtonii Morphotype B (see [2.] below). Although Shaw (2008: 22) notes that the illustration provided by Baldwin (1949: 344) does not allow placement of the plant in a given cultivar of the nothospecies, we speculate that the triploid material investigated by Baldwin (1949) was derived from plants as figured in Houghton (1935: 44), i.e., K. × houghtonii Morphotype B. Separately, Resende (1956: 241–242) reported producing two hybrid races in K. × houghtonii; one sterile, an F 1 triploid, and the other fertile, F 1 and F 2 tetraploids. It is therefore likely that K. × houghtonii Morphotype A rather represents the tetraploid race of the nothospecies.
Plants of K. × houghtonii Morphotype A are very invasive, so far naturalised in numerous mild-climate regions around the world, and due to their larger, K. daigremontiana -like leaves, produce significant quantities of bulbils along the margins. The cultivars K. × houghtonii ‘J.T. Baldwin’ (of which we consider K. × houghtonii ‘Jaws of Life’ to likely be a synonym) and K. × houghtonii ‘Garbí’, naturalised in Florida, USA, and Portugal and Spain, respectively ( Shaw 2008, Guillot Ortiz et al. 2014, Smith et al. 2015), belong to this group. Photos of plants of K. × houghtonii Morphotype A can be found at http://sven-bernhard.de/anzeige_bilder_en.htm, under “ K. × houghtonii B_255-10-93-30”.
Despite the fact that the one clone originally produced by Houghton very likely does not belong to Morphotype A, the type of K. × houghtonii clearly belongs to this Morphotype based on its size and leaf morphology. As stated above, it is this Morphotype that is typically found naturalised in various mild-climate regions, including in Florida, USA, from where the type was collected .
B. Kalanchoe × houghtonii Morphotype B ( Fig. 1E–F View FIGURE 1 )
Plants we regard as representative of Morphotype B have triangular-ovate leaf blades that are basally cuneate to subpeltate, and their large, inflated flowers are reminiscent of those of K. tubiflora . The corolla is usually orange rather than magenta, and the triangular-ovate leaves are much shorter than in Morphotypes A, C, and D, so giving the plants an overall smaller appearance when compared to the other Morphotypes, as well as both parents. Leaf arrangement transitions from decussate (as in K. daigremontiana ) in younger plants, to tricussate (as in K. tubiflora ) in mature plants. Leaves of plants of this group are adorned with darker purplish brown maculations both ab- and adaxially, and also along the petiole.
Plants of K. × houghtonii Morphotype B so far have been only documented with certainty in cultivation, and based on the ovate leaf blades, which are barely longer than the petiole, as figured in Houghton (1935: 44), we suggest that it is likely that Morphotype B, specifically, the cultivar K. × houghtonii ‘Hybrida’ ( Fig. 1E View FIGURE 1 ), was the one originally produced by Houghton. It is therefore likely that Morphotype B is the sterile triploid reported by Baldwin (1949: 343), who only reported the cytology of a single hybrid (but see Shaw 2008: 22).
Other than K. × houghtonii ‘Hybrida’, the first cultivar in K. × houghtonii to be artificially produced and described, the variegated K. × houghtonii ‘Pink Butterflies’ ( Fig. 1F View FIGURE 1 ), also belongs to K. × houghtonii Morphotype B and was likely vegetatively selected from K. × houghtonii ‘Hybrida’. Probable renamings of K. × houghtonii ‘Pink Butterflies’ include: K. × houghtonii ‘Pink Teeth’, K. × houghtonii ‘Pink Sparkler’, and K. × houghtonii ‘Fujicho’.
Perhaps as a result of their sterility, but more likely because of their smaller leaves that inevitably produce fewer bulbils per leaf, plants of Morphotype B appear to be less invasive than those of Morphotype A, though material representative of Morphotype B has become naturalised in some places, for example in Spain.
C. Kalanchoe × houghtonii Morphotype C ( Fig. 1G View FIGURE 1 )
Plants representative of Morphotype C have linear leaf blades that are attenuate apically and basally, and the large, inflated flowers are reminiscent of those of K. tubiflora . The corolla is usually orange, and the plants transition relatively quickly from a decussate to a tricussate leaf arrangement. Plants of this Morphotype are therefore hardly distinguishable from K. tubiflora , especially when grown under strong solar irradiation, which results in the linear leaves becoming more terete rather than more lanceolate. Leaves of plants of this group are generally only purplish brown-maculate abaxially and along the petiole.
To our knowledge, this Morphotype has yet to become naturalised successfully in foreign habitats, perhaps as a result of its leaf blade being shorter compared to its long petiole, bearing fewer bulbils per leaf. The cytology and fertility of material of Morphotype C are not known. Plants of this Morphotype exist in several Botanical Gardens that we visited, and also seem to occur naturally in Madagascar, for example along the Onilahy River, where the ranges of K. daigremontiana and K. tubiflora intersect [see for example https://www.inaturalist.org/observations/70546264]. It is not known if the naturally occurring plants of this Morphotype are transitional between the two parent species or whether they represent a natural hybrid between them. Photographic images of plants of this Morphotype in culture can be found at http://sven-bernhard.de/anzeige_bilder_en.htm, under “ K. × houghtonii HD_141070”. Due to their rarity in cultivation, no cultivar names exist for selections of K. × houghtonii Morphotype C.
*When grown under the same conditions, no difference can be observed between the two cultivars.
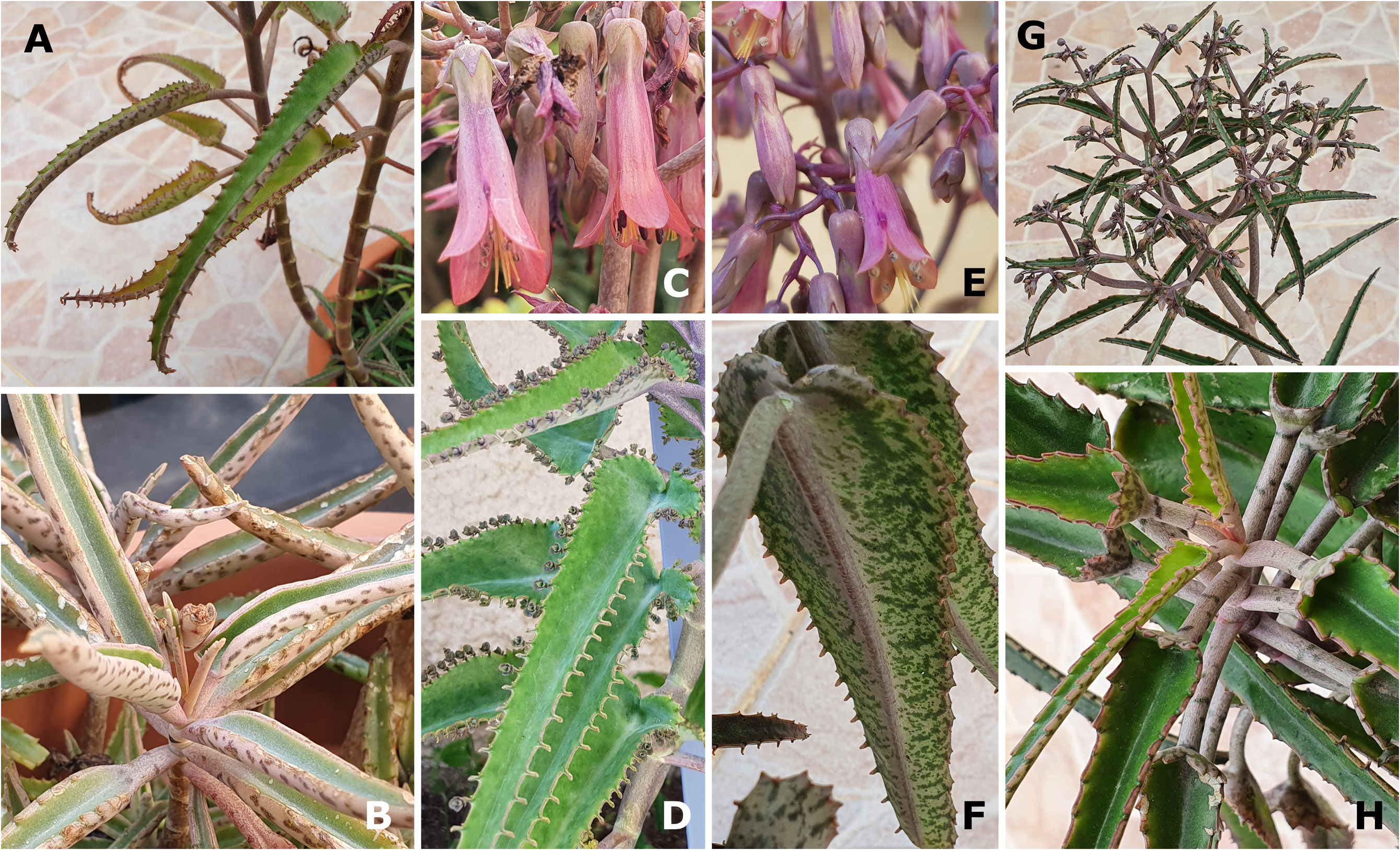
D. Introgressed K. daigremontiana / K. × houghtonii Morphotype D ( Fig. 2 View FIGURE 2 ; see also Smith & Shtein 2021: 246, Fig. 2C View FIGURE 2 )
Plants representative of Morphotype D have apically long-attenuate, lanceolate leaf blades that are auriculate to peltate basally, and the small, funnel-shaped flowers are reminiscent of K. daigremontiana . The corolla is usually pink-purple, rather than magenta-red (as in Morphotype A) or orange (as in Morphotypes B and C), and the leaves are much larger in mature plants, resulting in confusion between material of Morphotype D and K. daigremontiana . A further similarity to K. daigremontiana is the leaf arrangement that does not transition to tricussate—in plants of Morphotype D leaf arrangement generally remains decussate ( Smith & Shtein 2021: 246, Fig. 2C View FIGURE 2 ) or becomes alternate as flowering approaches [https://www.inaturalist.org/observations/12919993]. Young plants of Morphotype D are generally very similar in appearance to representatives of Morphotype C in that they have linear, though almost entire, leaves, while mature plants are hardly distinguishable from K. daigremontiana , as a result of their large, often peltate, leaves.
Considerable morphological variation exists in K. × houghtonii Morphotype D, with material having been observed that ranges from almost indistinguishable from K. daigremontiana (e.g., Fig. 2A–D View FIGURE 2 ), to plants more clearly transitional between K. daigremontiana and K. tubiflora , so highlighting the possibility that the material is indeed rather of hybrid origin [https://www.inaturalist.org/observations/12919993]; either K. × houghtonii or K. daigremontiana is variously applied to such transitional forms. To our knowledge, Morphotype D has yet to become naturalised successfully in foreign habitats, and the cytology and pollen and seed fertility of these plants are not known. Plants of K. × houghtonii Morphotype D exist in several Botanical Gardens that we visited, and also seem to occur naturally in Madagascar, for example in Toliara and along the Onilahy River, where the ranges of K. daigremontiana and K. tubiflora intersect [e.g., https://www.inaturalist.org/observations/70546286]. Again, it is not known if the plants of Morphotype D that occur naturally in Madagascar are transitional between the species ( K. daigremontiana and K. tubiflora ) or represent natural hybrids between them. Photographs of plants of Morphotype D in cultivation can be found at http://svenbernhard.de/anzeige_bilder_en.htm, under “ K. daigremontiana SSZ _96-2255-0”, a clone collected in 1996 from Toliara, Madagascar, by Dieter Supthut†, at the time of the Städtische Sukkulenten Sammlung, Zürich.
Plants referable to K. × houghtonii Morphotype D seem to arise occasionally in cultivation, for example, ISI 2007- 25 K. ‘Parsel Tongue’ ( Fig. 2E–H View FIGURE 2 ), a cultivar distributed by the International Succulent Institute through the Huntington Botanical Gardens, Pasadena, California, USA ( Trager 2007). Kalanchoe ‘Parsel Tongue’ is morphologically very similar to K. daigremontiana , except for its leaves that are apically long-attenuate; the funneliform-peltate (rather than saddle-shaped-peltate) leaf blade base; the finely-maculate abaxial leaf blade colouration; and the alternate leaf arrangement when mature. It is possible that this cultivar represents a back-cross of K. daigremontiana with a fertile cultivar of K. × houghtonii, perhaps K. × houghtonii ‘J.T. Baldwin’.
Summary:—Our studies and observations confirm that K. daigremontiana and K. tubiflora hybridise with ease, and even in their natural habitat some transitional or hybrid forms are present where the species grow sympatrically. Such forms that grow naturally in Madagascar are morphologically more diverse than the forms of K. × houghtonii that arose in cultivation. However, thus far only artificially created forms show strong invasive tendencies. Furthermore, many of the naturally occurring transitional or hybrid forms appear very similar to either K. daigremontiana or K. tubiflora , suggesting rather limited introgression. Nevertheless, where the natural geographical distribution ranges of K. daigremontiana and K. tubiflora overlap, several vegetative and reproductive morphological characters show considerable variation ( Table 1 View TABLE 1 ).
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| R |
Departamento de Geologia, Universidad de Chile |
| FLAS |
Florida Museum of Natural History, Herbarium |
| FTG |
Fairchild Tropical Botanic Garden |
| NY |
William and Lynda Steere Herbarium of the New York Botanical Garden |
| SD |
San Diego Natural History Museum |
| USF |
University of South Florida |
| A |
Harvard University - Arnold Arboretum |
| K |
Royal Botanic Gardens |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Kalanchoe × houghtonii Ward (2006: 94)
| Shtein, Ronen, Smith, Gideon F. & Ikeda, Jun 2021 |
Bryophyllum × houghtonii (D. B . Ward)
| Forster 2006: 383 |
Bryophyllum tubimontanum ’
| Houghton 1935: 44 |
Kalanchoe × hybrida HORT.
| Adanson 1763 |