Chamaepinnularia salina Beauger, C.E.Wetzel, Allain & Ector, 2022
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.538.1.5 |
|
DOI |
https://doi.org/10.5281/zenodo.6333082 |
|
persistent identifier |
https://treatment.plazi.org/id/0A107136-FFA7-FFB7-FF5A-FE7934CA385D |
|
treatment provided by |
Plazi |
|
scientific name |
Chamaepinnularia salina Beauger, C.E.Wetzel, Allain & Ector |
| status |
sp. nov. |
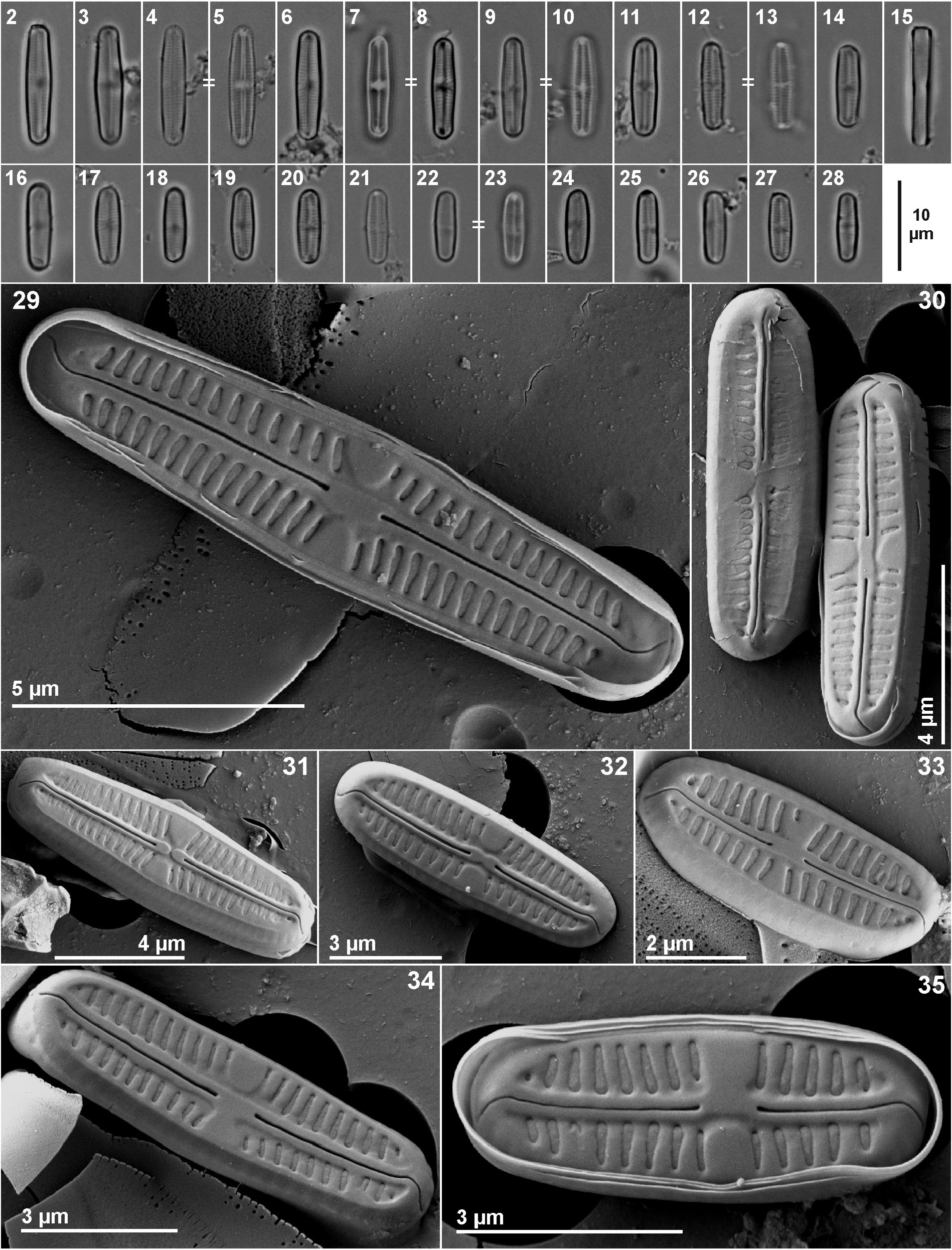
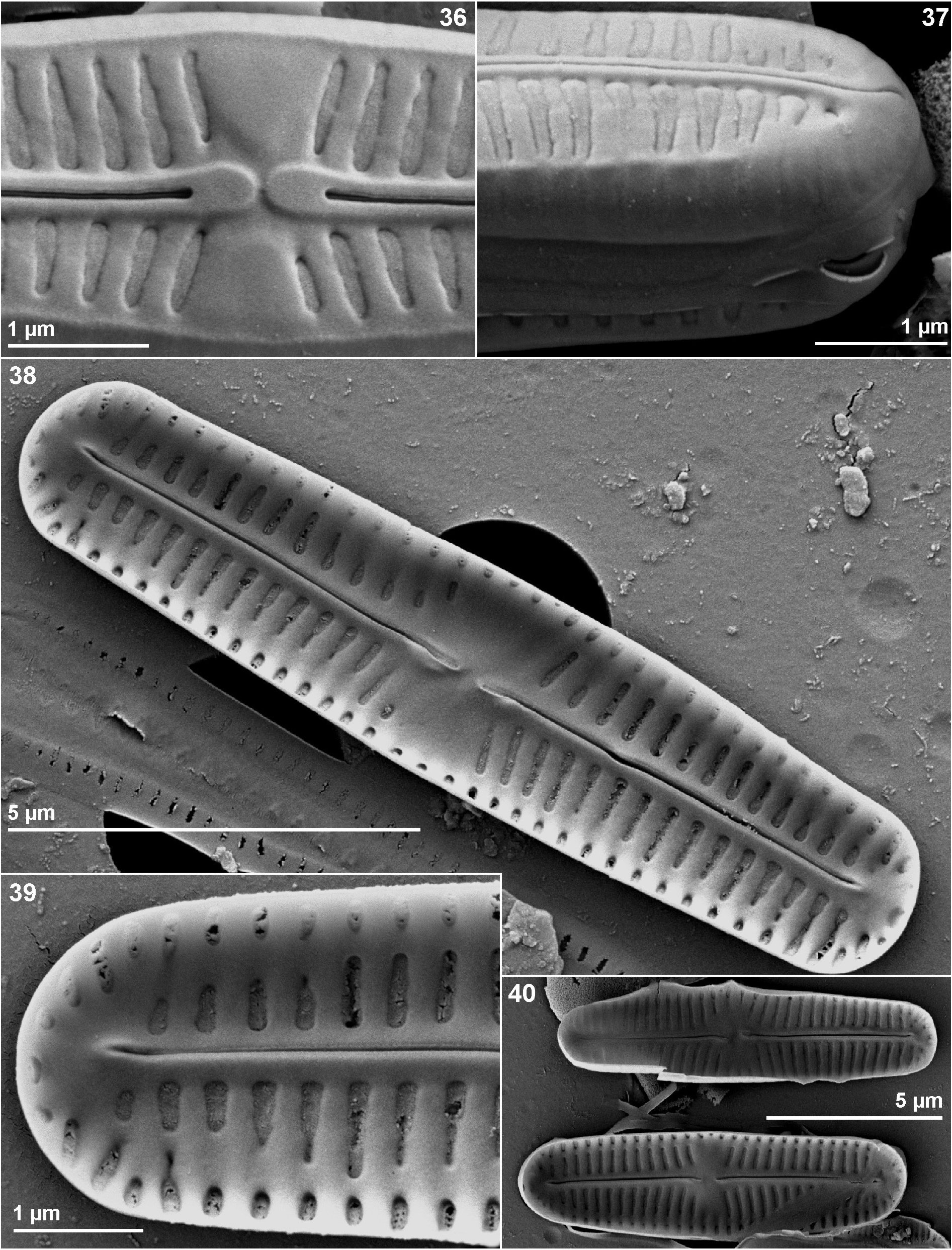
Chamaepinnularia salina Beauger, C.E.Wetzel, Allain & Ector sp. nov. ( Figs 2–40 View FIGURES 2–35 View FIGURES 36–40 )
Light microscopy observations ( Figs 2–28 View FIGURES 2–35 ): Valves linear with almost parallel margins (the longest individuals slightly inflated in the middle). Broadly rounded to slightly rostrate–capitate apices ( Figs 2–28 View FIGURES 2–35 ). Valve dimensions (n = 35): length 7–16 µm, width 2–3.2 µm. Axial area lanceolate, central area rectangular, forming a rather broad fascia. Striae parallel to weakly radiate near the central area, 30–40 in 10 µm.
Scanning electron microscopy observations ( Figs 29–40 View FIGURES 2–35 View FIGURES 36–40 ): Raphe straight (exceptionally slightly curved in the middle), filiform located on an elevated sternum and central area a rectangular fascia ( Figs 29–35 View FIGURES 2–35 ). Axial area narrow. Virgae between striae developed ( Fig. 36 View FIGURES 36–40 ). Raphe with straight, slightly expanded central endings and terminal fissures hooked in the same direction, continuing onto the mantle. Central endings expanded, drop-like ( Fig. 29 View FIGURES 2–35 ). Striae composed of two large areolae, one located on the valve face and a second on the mantle, separated by a silica line at the valve face/mantle junction, covered externally by porous hymenes, visible also internally ( Figs 36–39 View FIGURES 36–40 ). Striae sometimes slightly depressed ( Figs 36–37 View FIGURES 36–40 ). Around the valve apices, areolae on the mantle continuous externally and internally ( Figs 38–39 View FIGURES 36–40 ). Internally, terminal fissures slightly deflected, terminating in a slightly swollen hyaline area ( Fig. 39 View FIGURES 36–40 ).
Type: — FRANCE. Mirefleurs : Sail spring, sample S10_BA_ 201214, 334 m a.s.l., E716182.68, N6509572.63 (Lambert 93), collection date 20 December 2014 (designated here, holotype: CLF!, slide no. CLF103304 View Materials , here depicted in Figs 7–8 View FIGURES 2–35 ; isotypes: BR! slide no. 4649) .
Etymology: — The new species is named after the mineral and salty spring where it was discovered, Sail spring, situated in the French Massif Central.
Associated diatom taxa: — The type population of Chamaepinnularia salina was found in Sail spring associated with other taxa. In December 2014, the two co-dominant taxa were Planothidium frequentissimum ( Lange-Bertalot 1993: 4) Lange-Bertalot (1999: 282) (31.6%) and Chamaepinnularia salina (13.2 %). Crenotia thermalis ( Rabenhorst 1864: 107) Wojtal (2013: 81) represented 9.4 % of the community with Navicula veneta Kützing (1844: 95) (9.1%), Navicula sanctamargaritae (5.1%), Microcostatus sp. (4.6%), Nitzschia cf. liebetruthii (3.8 %), Gomphonema parvulum Kützing (1849: 65) (3.8 %), Pinnularia kuetzingii Krammer (1992: 104 , 173) (3.8%), Nitzschia vitrea G. Norman (1861: 7) (2.3 %), S urirella brebissonii Krammer & Lange-Bertalot (1987: 82) (2.0 %), Nitzschia inconspicua Grunow (1862: 579 , 580) (1.3 %). The other taxa (<1%) were Fallacia pygmaea ( Kützing 1849: 77) Stickle & D.G.Mann in Round et al. (1990: 668), Fragilaria famelica ( Kützing 1844: 64) Lange-Bertalot (1980: 749) , Nitzschia soratensis E.Morales & M.L.Vis (2007: 128) , Sellaphora labernardierei and lastly Pseudostaurosira sp.
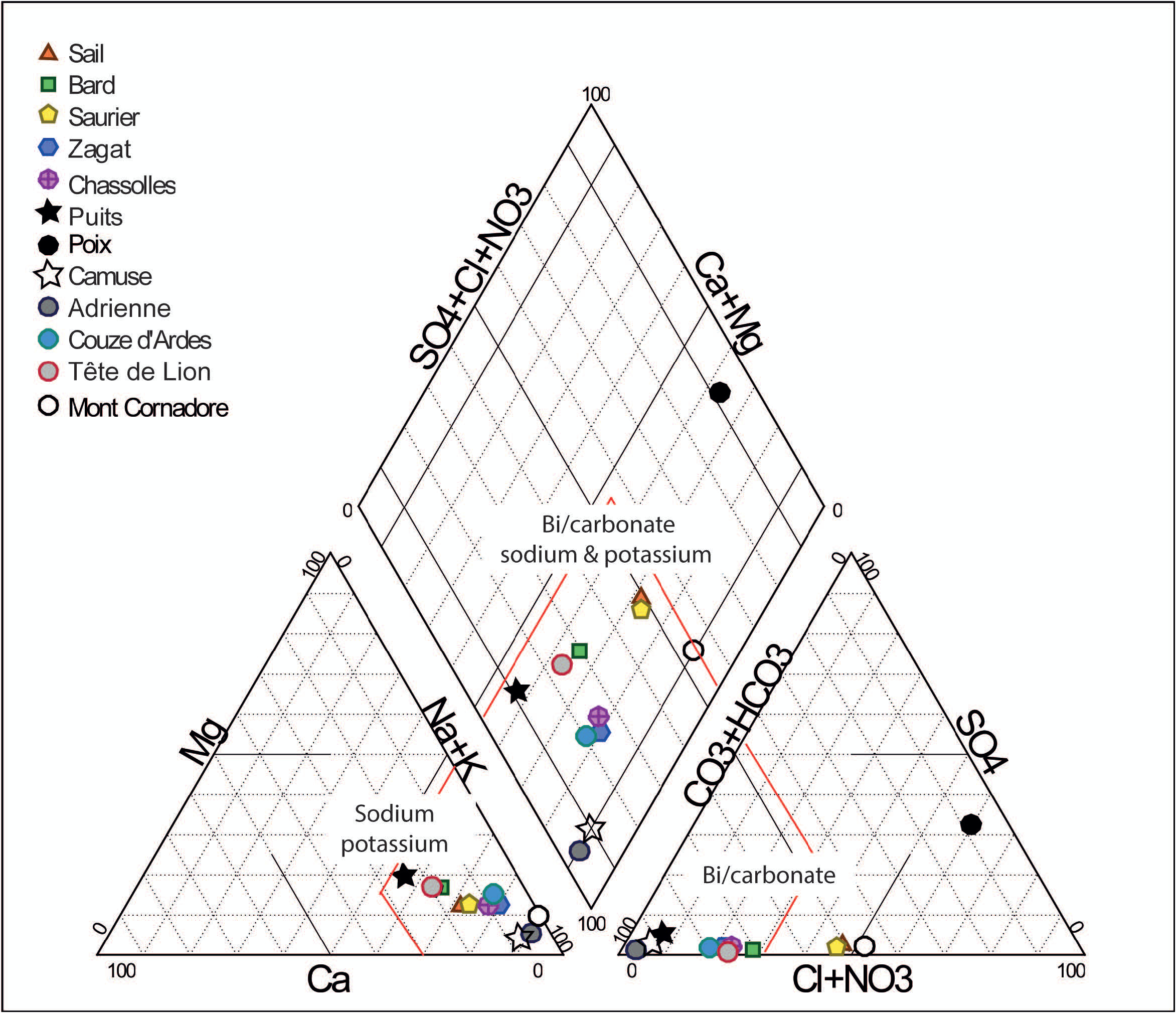
Physical and chemical environment of the species: — The Sail spring has a slightly acidic pH (6.8) and an elevated electrical conductivity (EC) level (more than 5000 μS cm-1). Water temperature is not high, in the range of 12.9 to 16.6°C for our measurements. The spring is rich in several major elements e.g. sodium, calcium and, the concentration in nitrate is lower less than 10 mg L- 1 ( Table 1 View TABLE 1 ). High trace elements concentrations such as boron, strontium, barium, manganese and arsenic are found in the 2018 sample ( Table 2 View TABLE 2 ). In October 2019, physical parameters and ionic concentrations are significantly different than the other periods of sampling, e.g. electrical conductivity, usually about 7600 µS. cm-1, has dropped to 5390 µS. cm-1 and chloride content has divided by two.
The Sail spring displays a very peculiar pattern regarding radioactivity: radon concentration in water varies significantly along the year and can reach significant levels up to 1000 Becquerels per liter. Indeed, the equivalent doses recorded (in nanoSieverts per hour) were on average 150 nSv h-1 and 270 nSv h-1 when measuring at 1-meter height above the spring and at the water level respectively. These values are in agreement with the mean background values of Auvergne region (150–200 nSv h-1) without indicating any significant radioactive signature. The water sample collected for gamma-spectroscopy measurements showed a significant Radon activity of 1006.1 ± 1.6 Bq L- 1.
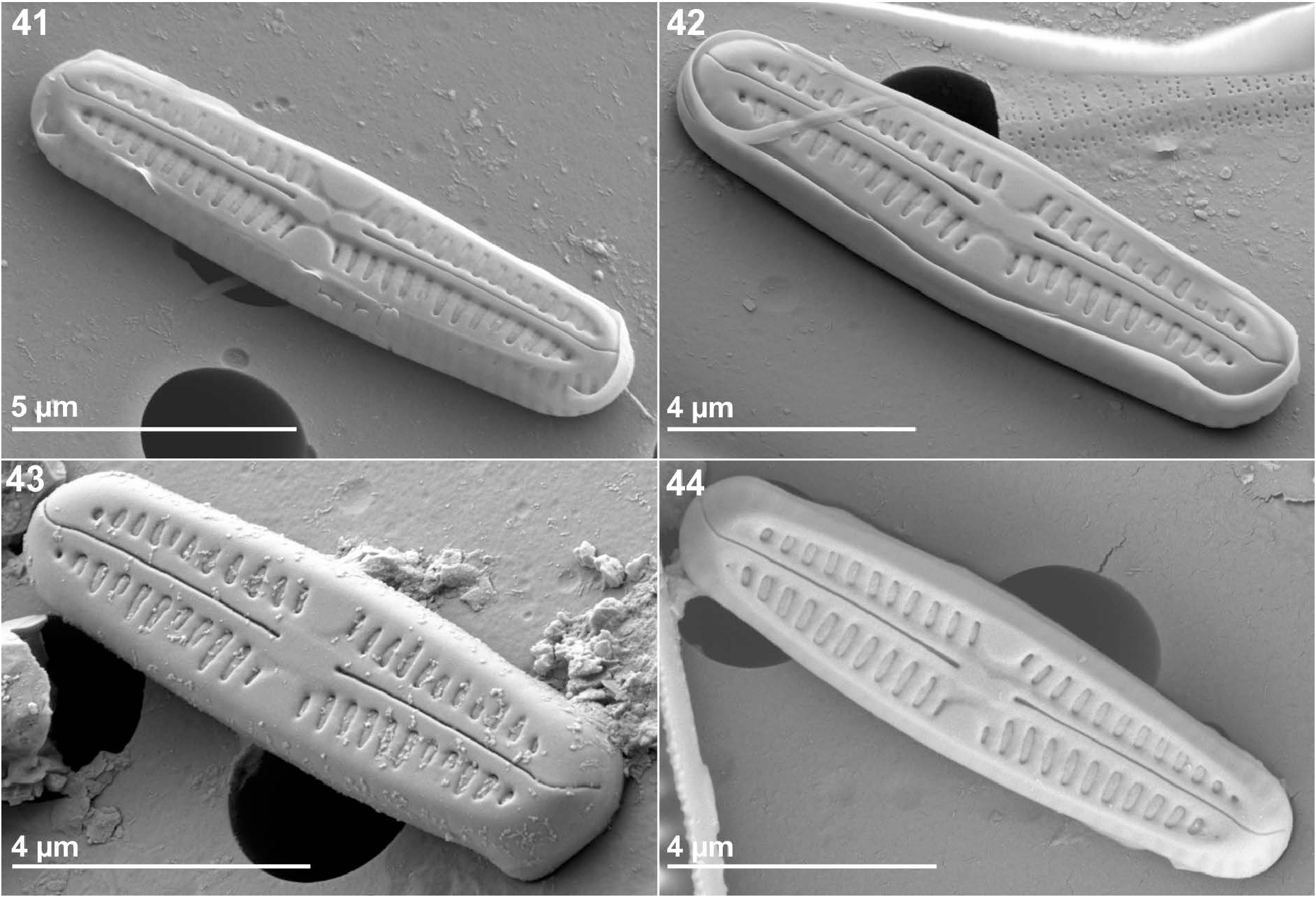
Chamaepinnularia salina was also present in each sample taken in 11 other springs of the French Massif Central ( Figs 41–44 View FIGURES 41–44 ; 45 View FIGURE 45 ). In these springs, the relative abundance of C. salina varied between 0.2% (one individual in Zagat and Tete de Lion springs) and 50.6% (Camuse spring). All the springs were characterized by high conductivity (between 1464 µS. cm-1 and 7240 µS. cm-1) and by high sodium and potassium contents ( Fig. 45 View FIGURE 45 ). Major anions are bicarbonates except for the Poix spring enriched in sulphate. For Camuse spring, where rock was brushed, C. salina was dominant (50.6%) associated with Humidophila gallica (W. Smith 1857: 11) R.L.Lowe, Kociolek, Q.You, Q.Wang & Stepanek (2017: 281) (30.8%). For Camuse spring, EC was less than 5000 μS cm-1 and ionic concentrations were lower than in Sail spring.
When collecting diatoms on mud, in November 2018, in Sail spring, only two individuals were observed and in October 2019, seven individuals were present. In June 2020, there were as many individuals (1.5%) in both samples (mud and stones). In the 11 other springs, C. salina was present both on stone and sediment substrates (the dominant substrates taken at each spring).
| CLF |
Institut des Herbiers Universitaires de Clermont-Ferrand |
| BR |
Embrapa Agrobiology Diazothrophic Microbial Culture Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |