Etmopterus sheikoi ( Dolganov, 1986 )
|
publication ID |
https://doi.org/ 10.26107/RBZ-2024-0002 |
|
publication LSID |
lsid:zoobank.org:pub:DF32BB8A-0012-4583-BAB8-4A79C039F157 |
|
persistent identifier |
https://treatment.plazi.org/id/0B5687CE-B95E-881A-2BEF-F90A1EE1511F |
|
treatment provided by |
Felipe |
|
scientific name |
Etmopterus sheikoi ( Dolganov, 1986 ) |
| status |
|
Etmopterus sheikoi ( Dolganov, 1986)
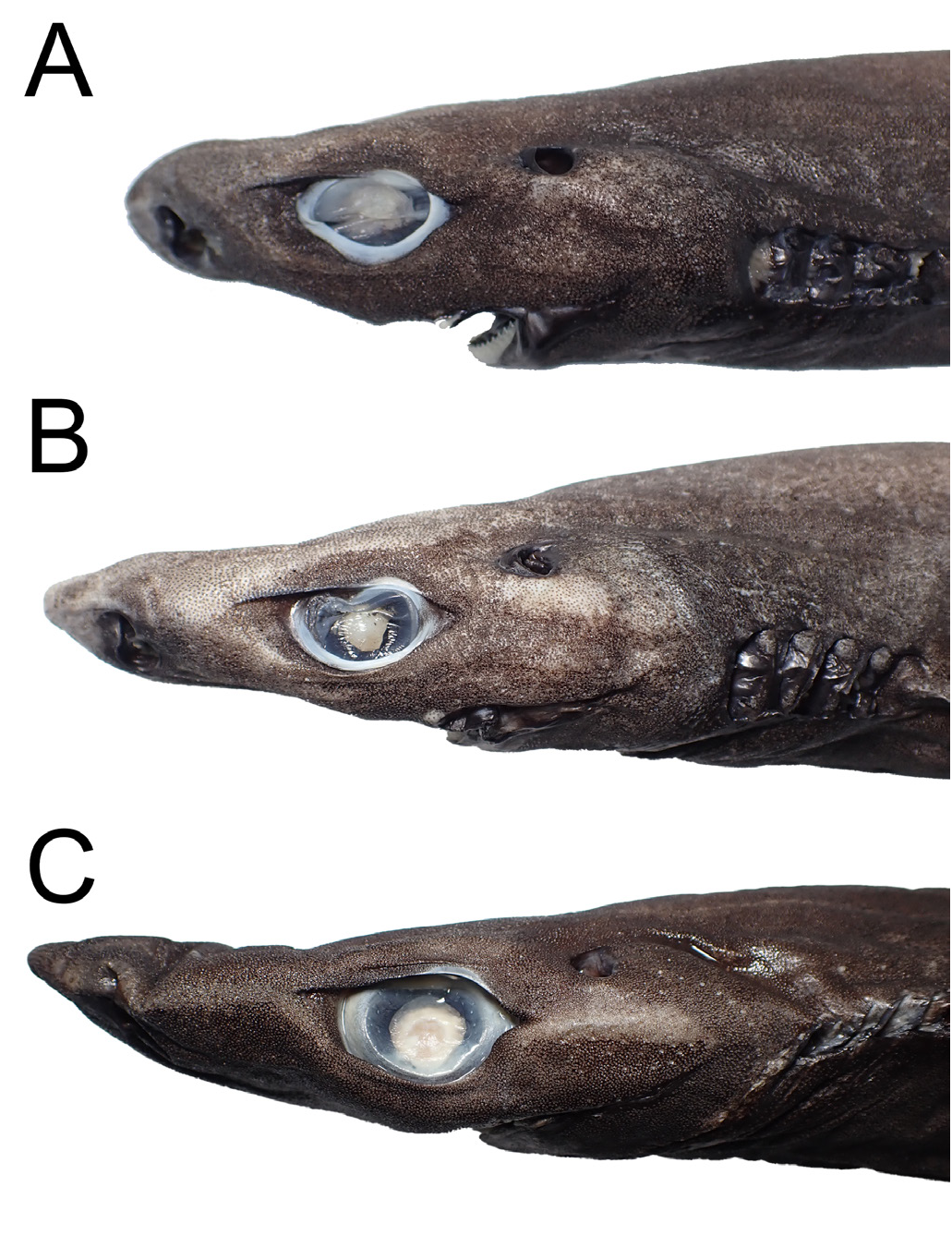
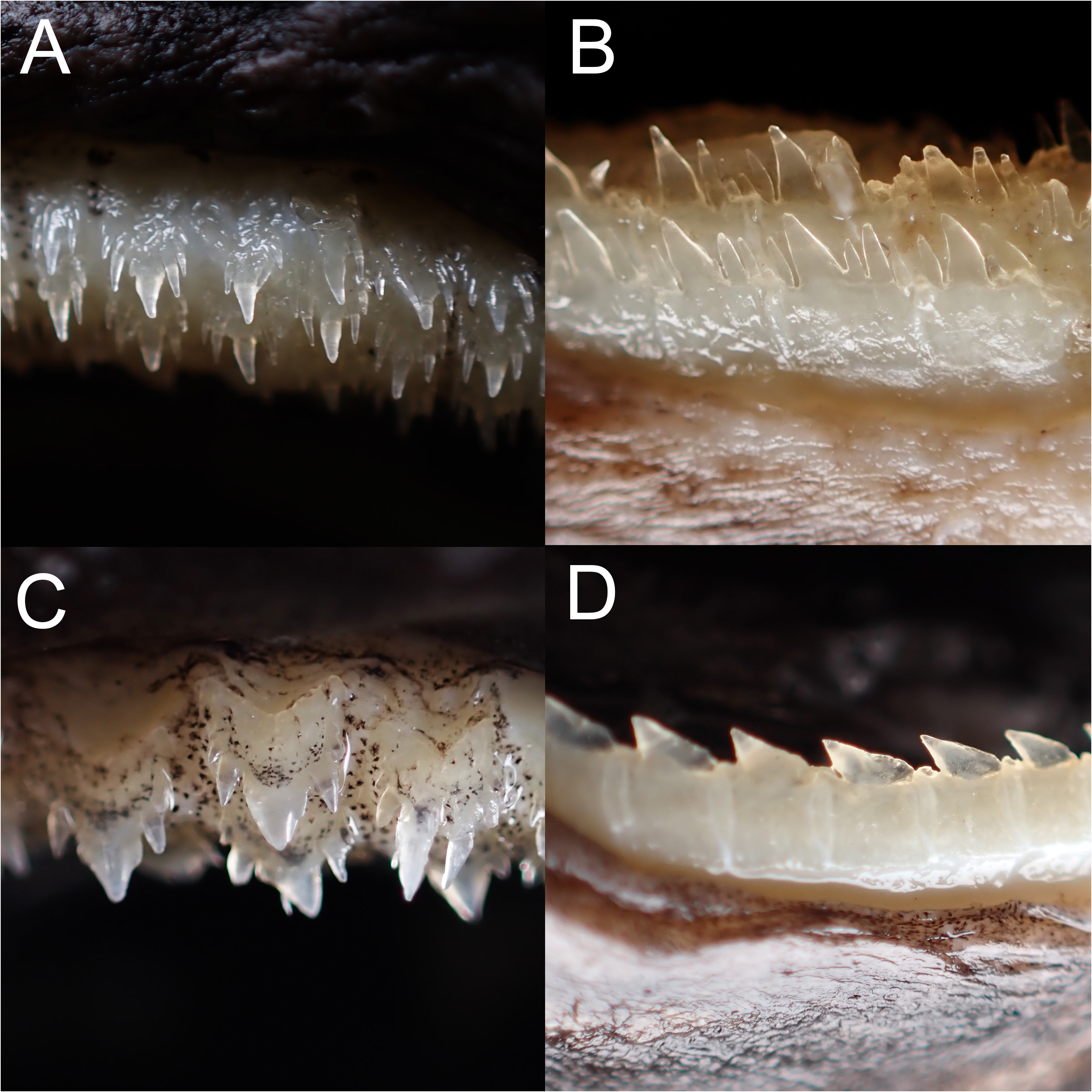
Rasptooth dogfish ( Figures 3C View Fig , 5B View Fig , 6B, D View Fig , 8–11 View Fig View Fig View Fig View Fig ; Tables 1–3)
Centroscyllium sheikoi Dolganov, 1986: 150–152 (holotype ZIN 46199 View Materials , Kyushu-Palau Ridge , Southern Japan).
Miroscyllium sheikoi Shirai & Nakaya, 1990: 355 View in CoL (new combination; description after Dolganov, 1986)
Centroscyllium sp. a. Nakaya in Okamura et al. (1982): 46, fig. 10
Etmopterus sp. Nakaya in Okamura et al. (1982): 52, fig. 15 Diagnosis. A moderately large species of Etmopterus differing from all other congeners, except E. lii , by having a combination of multicuspid lower jaw teeth in mature males, flat and frustum-shaped denticles, and elongated anterior and posterior lateral flank markings. It differs from E. lii by different aspects (see diagnosis in E. lii ).
Description based on Taiwanese specimens. Measurements are listed in Table 1. Values are expressed as a percentage of total length (TL).
Body fusiform ( Fig. 9 View Fig ), trunk sub-cylindrical, width 63.0– 125.6 % height; abdomen usually longer than lower caudal peduncle, pectoral-pelvic space 102.1–135.6 % (89.3 % in one immature male ASIZP0081757) pelvic-caudal space; head conical, length 22.6–27.2 % TL, rather depressed, height 67.4–90.1 % width. Snout very long, preorbital length 8.0–10.3 % TL, 34.5–40.9 % head length, 151.6–247.1 % orbit length; snout narrowly pointed in both dorsal and lateral view. Eyes oval, orbit width 46.4–77.8 % height; orbits with both anterior and posterior notches; eyes narrowly spaced, interorbital width 62.0 % 85.5 % head width, orbit length 53.4–78.8 % interorbital width. Spiracles small, reversed ‘D’-shaped, length 21.0–33.2 % orbit length, 3.8–5.6 % head length. Nostrils oblique, length 30.0–89.7 % internarial width, 29.1–57.6 % orbit length; anterior nasal flap narrowly triangular, tip reaching the nasal opening, length 22.9–61.0 % nostril width. Gill openings small, slightly oblique, intergill length 3.8–5.7 % TL, gill-slits height 0.7–1.9 % TL. Mouth broad, length 85.9–127.2 % width, nearly straight.
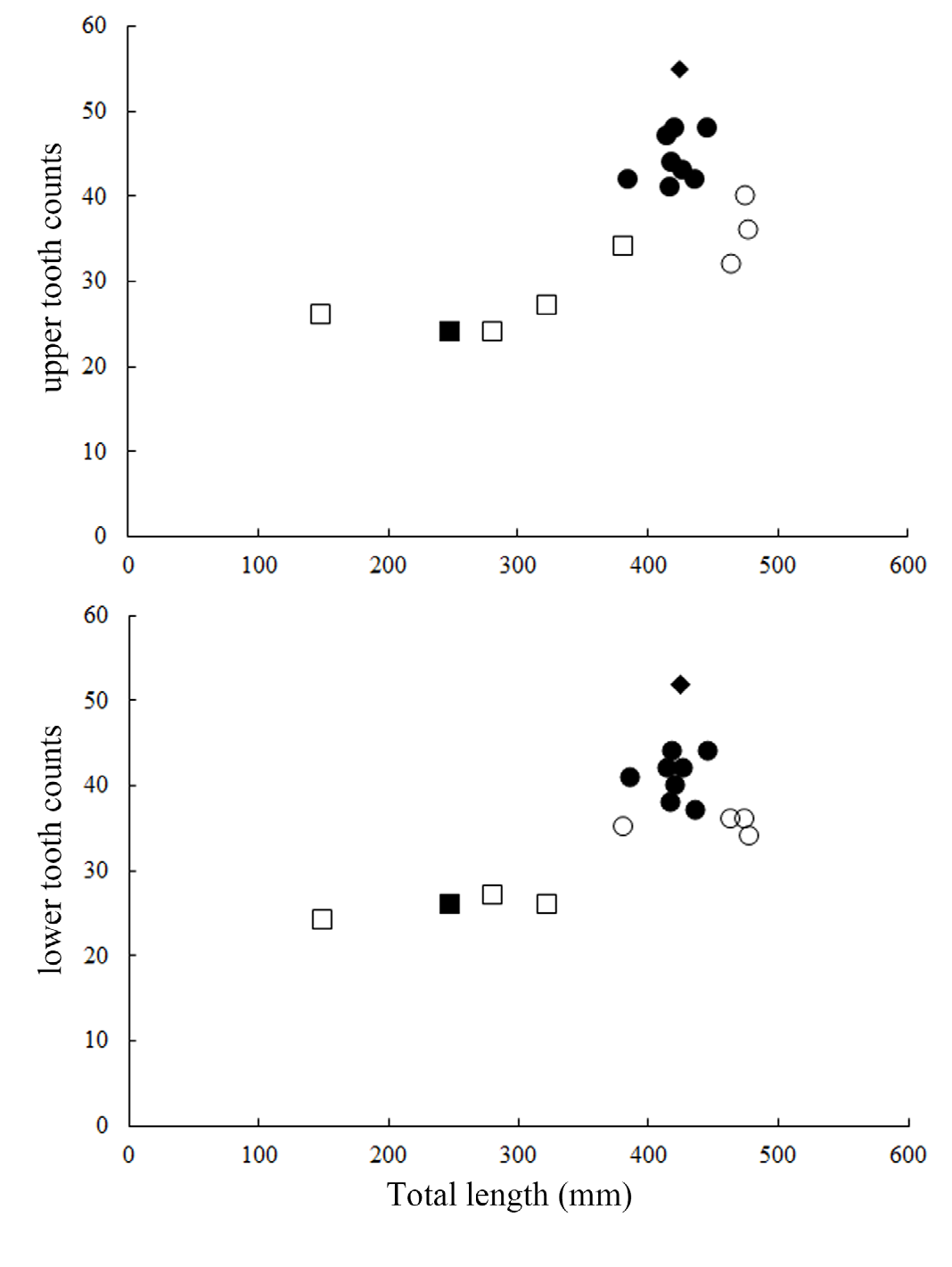
Teeth dissimilar in upper and lower jaw, with prominent ontogenetic and sexual dimorphism ( Fig. 9 View Fig ); upper teeth multicuspid, in three functional series, very small, central cusp rather slender; immature males and females with two cusplets on each side of the cusp of upper teeth, while mature males have three to four cusplets; the third pair of cusplet (counted from mesial to distal side) longest, length about half to two-third of the central cusp; teeth in lower jaw unicuspid in immature individuals, in three series, one functional; lower teeth blade-like, with strongly oblique cusp; the cusps of lower teeth of mature males are flanked with two to three straight, erect cusplets on each side, the outer one minute; the cusps of lower teeth in one mature female (ASIZP0081763) are flanked with one minute cusplet on each side, while other mature females are unicuspid. Tooth count of upper jaw 24–48, lower jaw 26–44, total count 50–92.
First dorsal fin moderate, with a broadly angular apex, length of first dorsal fin 7.7–11.0 % TL, origin within to just posterior to pectoral-fin free rear tip; pre–first dorsal fin length 130.6–194.1 % interdorsal space; first dorsal–fin spine 125.1–194.5 % first dorsal-fin height. Second dorsal fin much larger than first dorsal fin, first dorsal-fin height 46.9–75.0 % second dorsal-fin height; apex narrowly angular, posterior margin especially concave, free rear tip moderately elongated; second dorsal-fin length 11.0–13.0 % TL, interdorsal space 130.5–206.5 % dorsal-caudal space; second dorsal–fin spine long and curved; second dorsal-fin origin well posterior to insertion of pelvic fins. Interdorsal space 67.2–107.3 % pre– pectoral length. Pectoral fins moderate, length 7.0–11.2 % TL, with sharply pointed free rear tips, base narrow, 44.3–58.7 % pectoral-fin length, posterior margin slightly concave to slightly convex. Pelvic fin triangular, height 27.0–42.0 % length. Clasper of mature males rather long, inner length 61.6–74.3 % pelvic-fin length. Caudal fin elongated, dorsal length 19.3–26.1 % TL; caudal fork moderately developed, lower postventral margin 13.4–38.4 % upper postventral margin; terminal lobe somewhat broad.
Dermal denticles frustum-shaped, small, flat, extremely closely spaced, giving a smooth texture of the skin, not in defined rows; denticles present on underside of snout, except for a narrow area around mouth; underside of gill slits with a V-shaped naked area, connecting gill slits between both lateral sides; inner margin of fins with very narrow naked area, except for pectoral fin with a large naked area; denticles present on fin bases, scarcely present on ceratotrichia.
Luminescent markings on head distinct when fresh, a line originates from above nostril extend across a horizontal mid-orbit towards lower gill slits; head dorsal surface with a single line of dot-like markings, extending mid-dorsally from about the level of anterior fontanelle to the second dorsal-fin origin; ventral surface of pectoral fin with a triangular marking, sometimes arched, the tip not reaching the origin of pectoral-fin ceratotrichia; dash-like markings present on lateral side. Pelvic-fin flank markings well defined, with elongated anterior and posterior branch; anterior branch rather long and thick, length 5.6–11.3 % TL, slightly curved, extending above pelvic–fin origin; posterior branch straight, very thick and shorter than anterior branch, with a square-like tip, length 35.5–91.1% length of anterior branch, width 1.5– 2.3 % TL, not extending beyond second dorsal-fin free rear tip; base of flank marking broad, base length 3.8–7.2 % TL, origin well anterior to second dorsal-fin origin. Infracaudal marking extending from the pelvic-fin flank marking base to about the same level of the posterior marking tip, not connecting to the caudal-fin base marking. Caudal-fin base marking thick and flat, rather long, originate well before lower caudal-fin origin, bifurcate after the origin, leaving a small black portion on the lower caudal-fin origin when viewed laterally. No central caudal-fin marking. Posterior caudal-fin marking very long, its length 7.8–11.3 % TL.
Vertebral counts: monospondylous 42–45, diplospondylous precaudal 14–20, caudal 23–31, precaudal 58–64, total 85–93.
Colouration. When fresh, body generally shiny purplish to dark grey, black ventrally; transition between lateral and ventral sides strongly demarcated. Dorsal midline without pale stripe; Markings on lateral side dot-like, prominent when fresh. Pectoral and pelvic fins generally translucent, with darker bases; dorsal fins mostly pale grey in two-third portions of ceratotrichia. Caudal-fin dorsal and postventral margins translucent, with a dark blotch covering from dorsal margin to mid-caudal fin, not extending to the upper postventral margin. A black blotch present between infracaudal and caudal-fin base marking. Caudal fin with a distinct black tip (terminal margin).
After preservation, body colouration slightly darker, yet most of the markings remain distinct ( Fig. 10 View Fig ). Transition between lateral and ventral sides becomes less demarcated. Blotches between infracaudal and caudal-fin base marking, and on mid-caudal fin usually become less discernable (usually fade). A white spot on cheek area emerges, which is not as prominent as in fresh condition.
Size. Up to 478 mm TL and 446 mm TL for females and males, respectively. Possibly attains 500 mm TL. The two specimens with length 149 mm TL have an umbilical scar, which should represent the approximate birth size.
Distribution. Known from the northwestern Pacific, off southern Japan ( Dolganov, 1986), northeastern and southwestern Taiwan, at depths of approximately 300– 500 m.
Biological note. The smallest mature female and male measured 464 mm TL and 386 mm TL, respectively. Length of full-term embryos range from 98 to 139 mm TL. Maximum size probably exceeds 500 mm TL. This species feeds mainly on myctophids and small macrourids, based on observations from some specimens (not retained, Ng, unpublished data).
Remarks. Compagno (1984) recognised a ‘slender, Etmopterus -like, long-nosed’ Centroscyllium from Japan based on Nakaya in Okamura et al. (1982). This specimen is now reidentified as E. sheikoi .
Although deeply clustered within the E. lucifer group, Straube et al. (2010) did not clearly assign E. sheikoi to any groups, as they considered the shape of pelvic-fin flank markings to resemble the typical shape found in species in the E. pusillus group. However, after examining additional material of this species, this marking actually comprises both elongated anterior and posterior branches (absent in species of E. pusillus group) ( Fig. 6B, D View Fig ), which is a typical character in the species of E. lucifer group. Therefore, we formally assign E. sheikoi into a member of the E. lucifer group based on morphological and genetic evidence. Similarly, E. sheikoi does not show a linear arrangement of dermal denticles, which further supports that the character ‘arrangement of denticles in linear rows’ cannot be considered a shared character in this group.
The specimens examined in this study are generally consistent with the original description of the holotype ( Dolganov, 1986), except for some morphometrics, which are substantially lower than the range of our specimens. For example, the prebranchial length is 9.1 % TL in the holotype (vs. 18.2–22.5 % TL in other specimens), which is obviously an error because it is even shorter than its preoral length (12.1 % TL), which is always shorter than prebranchial length in all elasmobranchs. The dorsal-fin length is also shorter than our specimens (D1 length 4.7 % TL in the holotype vs. 7.7–11.0 % TL; D2 length 7.1 % TL vs. 11.0–13.0 % TL). In the present study, the length of the dorsal fin was measured from the origin of the fin spine to the free rear tip end. However, Dolganov (1986) did not state the methods of measurement in detail, so we cannot exclude the possibility that only the fin portion was measured in the latter. Thus, the difference in dorsal-fin length may be attributed to different methodologies.
The holotype also has slightly fewer monospondylous vertebrae and slightly more diplospondylous trunk vertebrae than our specimens ( Table 1). Such subtle differences (1 vertebra each) can be reasonably considered as individual variations, which can be assessed when examining more specimens. In addition, the holotype has substantially more teeth (55/52 vs. 24–48/26–44). As the holotype was collected in Japan, the more numerous teeth may reflect geographical variations, as reported in other lanternshark species (e.g. E. pusillus, Shirai & Tachikawa, 1993 ).
Ontogenetic differences in tooth count were reported in some lanternshark species (e.g. E. spinax, Straube & Pollerspöck, 2020 ), where large individuals have more teeth than small individuals. In E. sheikoi , we also noted this phenomenon, with large individuals (> 382 mm TL) having more teeth (32–55/34–52) than small individuals (<323 mm TL) (24–27/24–27). Notably, mature males have more teeth than mature females (41–55/37–52 vs. 32–40/34–36) reflecting sexual dimorphism in tooth count, which is possibly the first documented case within the order Squaliformes.
The ontogenetic changes in morphology of teeth have been well described in Adnet et al. (2006). While reporting the strong ontogenetic heterodonty, the comparative materials in Adnet et al. (2006) were mature and immature males, therefore, the dentition and possible changes in dentition throughout the ontogeny of females are still unknown to date. Here, we document the first heterodonty in the lower teeth of mature females of E. sheikoi , observing mature females with a pair of cusplets on each side of the main cusp. The size of the cusplets is minute when comparing to the slender, erect ones in males. The ecological function of sexual and ontogenetic heterodonty in counts and morphologies maybe explained by intraspecific niche partitioning, in other words, that mature males and females may inhabit different depths encountering different prey taxa. In fact, mature females of E. sheikoi are much rarer than mature males in Taiwanese fishing ports based on intensive sampling efforts (Ng, unpublished data), which possibly suggests that mature females live in deeper waters where fishing activities rarely occur.
Comparisons. Etmopterus sheikoi is a unique species within the genus by having a remarkable long, narrowly pointed snout, which makes it readily distinguishable from most of its congeners. The combination of frustum-shaped denticles, thick posterior branch of the pelvic-fin and caudal-base markings, and multicuspid lower teeth in mature males, also make it distinctive among the E. lucifer group. It is most similar to Etmopterus lii but can be readily separated from it (see comparisons of E. lii above).
Etmopterus bigelowi also possesses a relatively long snout and frustum-shaped denticles, which looks quite similar to E. sheikoi at first glance. Nevertheless, E. sheikoi differs from the former by possessing a longer snout (34.5–40.9 vs. 23.6–32.8 % head length), a much shorter caudal-fin base marking, length 7.0–9.1% TL (vs. 13.6–18.0 % TL), a much longer posterior caudal-fin marking, length 7.8–11.3 % TL (vs. 2.3–3.7 % TL) an elongated posterior flank marking branches (vs. absent), multicuspid lower teeth in mature males (vs. unicuspid), a triangular pectoral-fin marking (vs. subrhombic-shaped), much fewer monospondylous centra (41–45 vs. 53–55), and much more diplospondylous trunk centra (14–21 vs. 7–12).
Materials examined. Etmopterus alphus (n=2): USNM 43291 About USNM , paratype, 282 mm TL, immature male , USNM 432492 About USNM , paratype, 315 mm TL, mature male, off Mozambique (18°14′ S, 37°31′ E), 472 m, 17 July 1994 GoogleMaps , R.W. Leslie ; Etmopterus bigelowi (n=11) : USNM 157835 About USNM , paratype, 422 mm TL, mature male, off Pensacola (29°13′ N, 87°54′ W), 458 m GoogleMaps , R / V Oregon , 13 March 1955 ; USNM 220331 About USNM , 8 paratypes, 157–222 mm TL, Gulf of Mexico , western Central Atlantic (29°01′ N, 88°59′ W), 403 m GoogleMaps , R / V Oregon ,
24 August 1962; USNM 220332 About USNM , 2 About USNM , paratypes, 250–347 mm TL, off Panama, Caribbean Sea (09°22′ N, 80°72′ W), 274 m , R / V Oregon , 30 May 1962; Etmopterus lucifer (n=1): EBFS-NG 00047 , 317 mm TL, mature male, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 19 November 2021; Etmopterus cf. molleri (n=1): EBFS-NG 00158 , 349 mm TL, mature female, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 21 June 2022; Etmopterus pusillus (n=1): EBFS-NG 00296 , 419 mm TL, mature male, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 12 June 2022; Etmopterus sheikoi (n=23): ASIZP0081746 View Materials , 6 View Materials , 98–139 mm TL, full-term embryos, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 20 October 2018; ASIZP0081747 View Materials , 418 mm TL, mature male, ASIZP0081748 View Materials , 419 mm TL, mature male, ASIZP0081749 View Materials , 437 mm TL, mature male, ASIZP0081750 View Materials , 427 mm TL, mature male, ASIZP0081751 View Materials , 446 mm TL, mature male, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 1 March 2022, J.- H. Hong; ASIZP0081752 View Materials , 282 mm TL, immature female, ASIZP0081753 View Materials , 323 mm TL, immature female, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 27 August 2021; ASIZP0081754 View Materials , 421 mm TL, mature male, off Daxi, northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 11 October 2021; ASIZP0081755 View Materials , 415 mm TL, mature male, off Daxi, northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 17 April 2021; ASIZP0081756 View Materials , 382 mm TL, immature female, off Donggang, southwestern Taiwan (ca. 22° N, 120° E), ca. 400 m GoogleMaps , 10 December 2021; ASIZP0081757 View Materials , 149 mm TL, immature male, off Daxi, northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 26 March 2021; ASIZP0081758 View Materials , 149 mm TL, immature female, off Donggang, southwestern Taiwan (ca. 22° N, 120° E), ca. 400 m GoogleMaps , 10 December 2021; ASIZP0081759 View Materials , 386 mm TL, mature male, off Daxi, northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 18 May 2022; ASIZP0081760 View Materials , 248 mm TL, immature male, off Donggang, southwestern Taiwan (ca. 22° N, 120° E), ca. 400 m GoogleMaps , 9 November 2022; ASIZP0081761 View Materials , 464 mm TL, mature female, off Donggang, southwestern Taiwan (ca. 22° N, 120° E), ca. 400 m GoogleMaps , 16 September 2022; ASIZP0081762 View Materials , mature female 478 mm TL, ASIZP0081763 View Materials , mature female 478 mm TL, off Daxi , northeastern Taiwan (ca. 24°90′ N, 122°00′ E), ca. 400 m , 12 June 2022.
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Etmopterus sheikoi ( Dolganov, 1986 )
| Ng, Shing-Lai, Liu, Kwang-Ming & Joung, Shoou-Jeng 2024 |
Miroscyllium sheikoi
| Shirai S & Nakaya K 1990: 355 |
Centroscyllium sheikoi
| Dolganov VN 1986: 152 |
Centroscyllium sp.
| Okamura O & Amaoka K & Mitani F 1982: 46 |