Taumacera Thunberg, 1814
|
publication ID |
https://doi.org/ 10.2478/aemnp-2019-0003 |
|
publication LSID |
lsid:zoobank.org:pub:6E92818A-CE7D-4809-854B-158FEA27AD4F |
|
DOI |
https://doi.org/10.5281/zenodo.5062665 |
|
persistent identifier |
https://treatment.plazi.org/id/0C4EEE5E-FFE0-015B-621E-F46496EC8D86 |
|
treatment provided by |
Felipe |
|
scientific name |
Taumacera Thunberg, 1814 |
| status |
|
Taumacera Thunberg, 1814: 48 (type species: Taumacera deusta Thunberg, 1814 , by monotypy).
Thaumacera Gemminger & Harold, 1876: 3560 (unjustified emendation of Thaumacera for Taumacera ).
Acroxena Baly, 1879b: 462 (type species: Acroxena nasuta Baly, 1879 , by original designation), syn. nov.
Doridea Baly, 1864: 236 (type species: Doridea insignis Baly, 1864 , by original designation); WEISE (1922): 108 (synonymized with Platyxantha ); REID (1999): 1 (synonymized with Taumacera ), synonymy confirmed.
Dorydea: CHAPUIS (1875) : 245 (subsequent incorrect spelling of Doridea Baly ).
Kinabalua Mohamedsaid, 1997b:132 (type species: Kinabalua antennata Mohamedsaid, 1997 , by original designation), syn. nov.
Metellus Jacoby, 1886: 63 (new substitute name for Neocharis Jacoby, 1881 nec Sharp, 1877); WEISE (1913): 231 (synonymized with Nacrea ); BRYANT (1923): 147 (synonymized with Nacrea ).
Nacrea Baly, 1886a: 29 (type species: Nacrea maculata Baly, 1886 , by original designation); JACOBY (1891):65 (synonymized with Metellus ).
Neocharis Jacoby, 1881: 448 (type species: Neocharis fulvicollis Jacoby, 1881 , by original designation). Preoccupied by Neocharis Sharp, 1877: 485 ( Coleoptera View in CoL : Eucnemidae View in CoL ).
Neochrolea Jacoby, 1887a: 117 (type species: Neochrolea cavifrons Jacoby, 1887 , by monotypy); MAULIK (1936): 580 (synonymized with Palpoxena ), syn. nov.
Paraenidea Laboissière, 1933: 66 (type species: Paraenidea azurea Laboissière, 1933 , by original designation); KIMOTO (1989): 202 (synonymized with Platyxantha ); REID (1999): 3 (synonymized with Palpoxena ), syn. nov.
Platyxantha Baly, 1864: 233 (type species: Platyxantha apicalis Baly, 1864 , by original designation); REID (1999): 1 (synonymized with Taumacera ).
Platyxanthoides Laboissière, 1933: 71 (type species: Platyxanthoides variceps Laboissière, 1933 , by original designation); GRESSITT & KIMOTO (1963):685 (synonymized with Paraenidea ); KIMOTO (1989): 202 (synonymized with Platyxantha ); REID (1999): 1 (synonymized with Taumacera ).
Xenarthra Baly, 1861: 298 (type species: Xenarthra cervicornis Baly, 1861 , by original designation), syn. nov.
Redescription (modified and extended based on REID (1999, 2001)). Body length 5.0–12.0 mm.
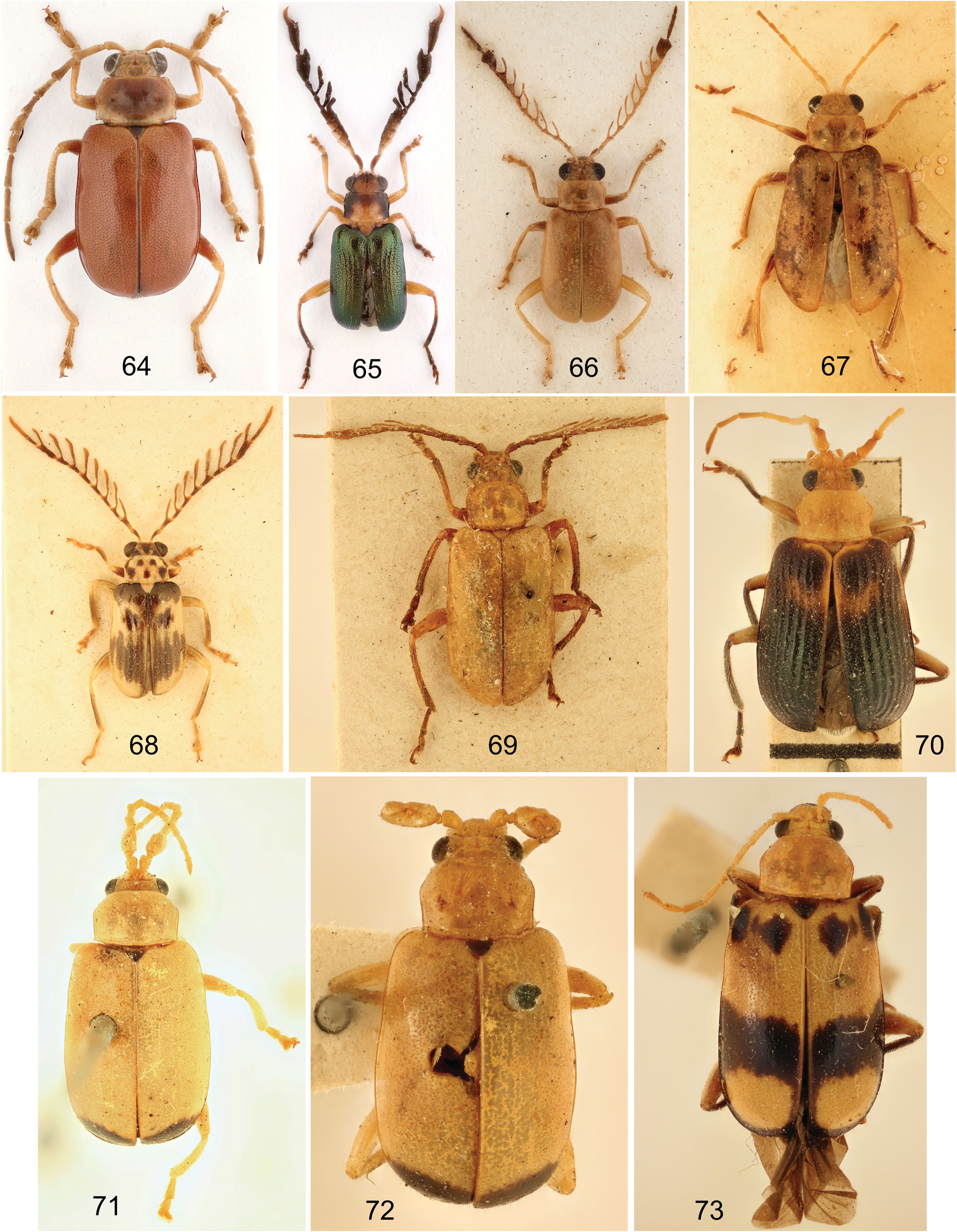
Males. Head. Eyes moderately large. Gena 0.2–0.4 times as long as eye. Anterior part of head not modified or modified (e.g., surface even or convex, dull, covered with punctures and wrinkles; shallowly concave; strongly excavated and modified; or head extremely thin in lateral view – Figs 43–54 View Figs 43–54 ). Labrum usually not modified or, rarely, enlarged (the nasuta -group). Frontal tubercles flat, broad, rectangular, usually with produced inner anterior angle, rarely transversely triangular. Penultimate maxillary palpomere not greatly swollen, apical palpomere conical.
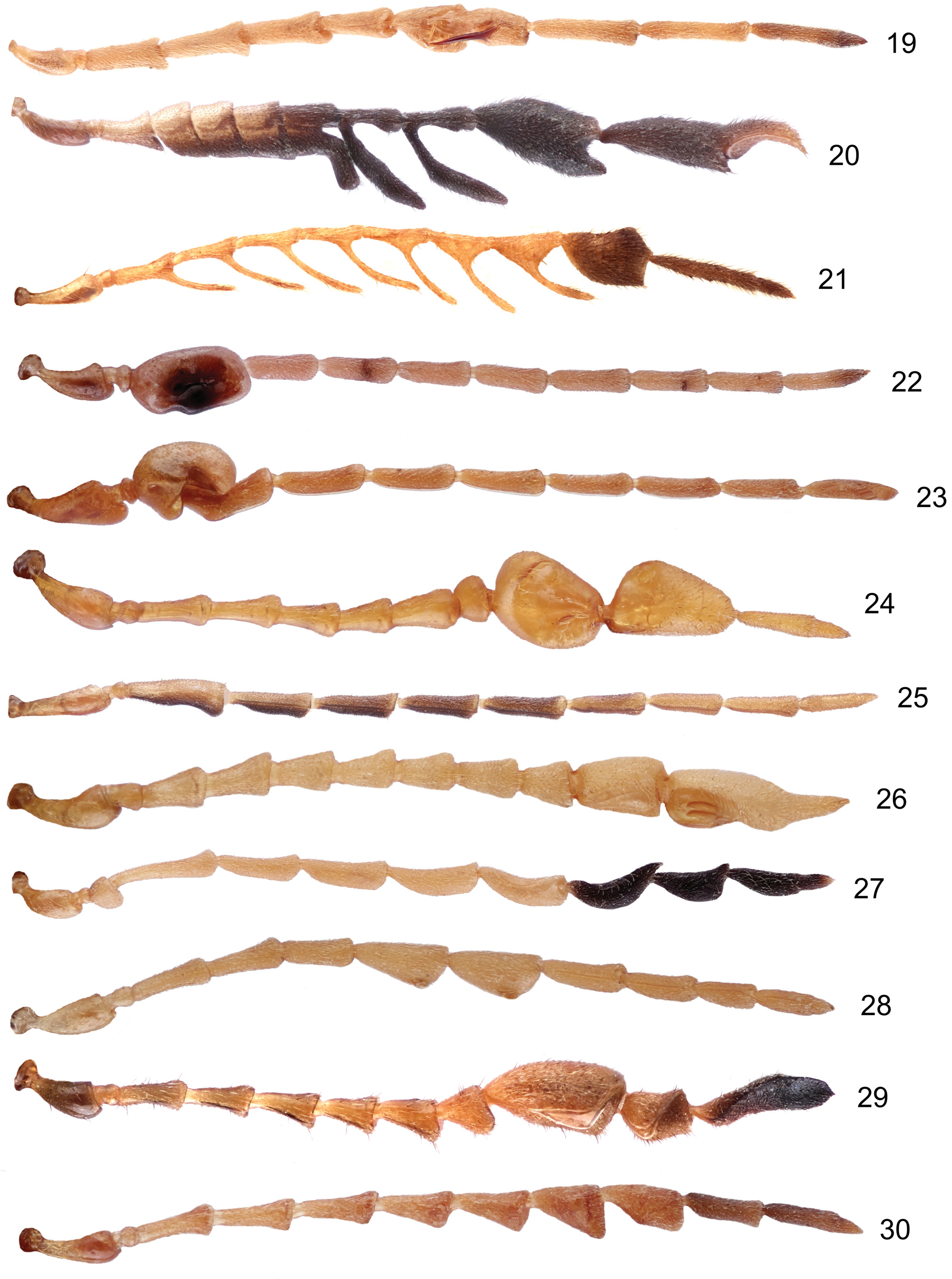
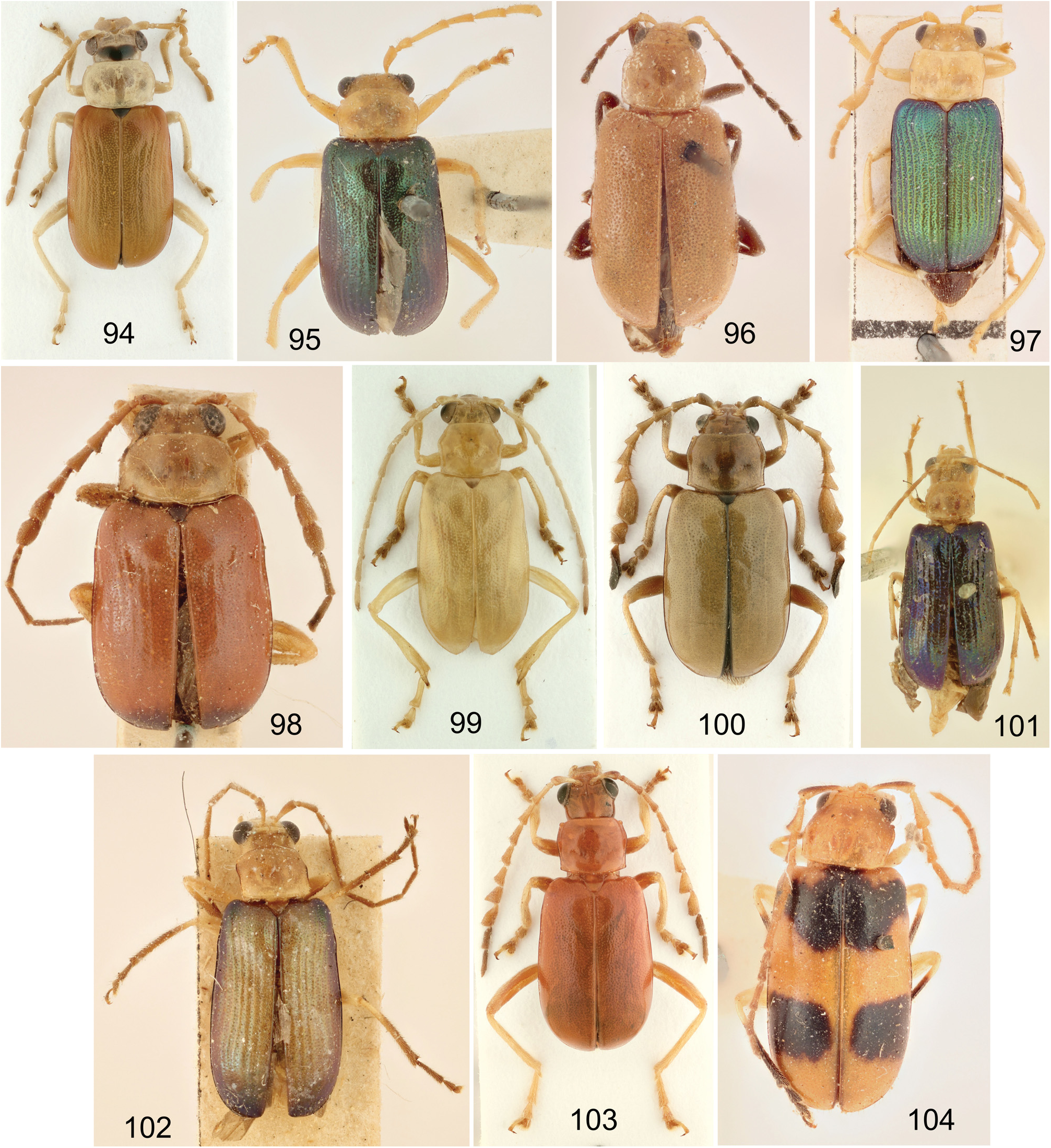
Antennae 11–segmented (except T. cervicornis with 12 antennomeres), slender with antennomeres relatively robust, often some antennomeres strongly modified (for antennal shape examples see Figs 19–30 View Figs 19–30 , 97–104 View Figs 94–104 ); antennomere II very short, III long, 3.5–6.0 times longer than II, 0.85–1.40 as long as I (usually subequal in lenght) and about 2.5–5.0 times as long as wide (cannot be applied for species with modified antennomere III). Antennomeres sometimes longitudinally ridged.
Pronotum transverse, 1.2–1.6 times as wide as long, broadest at middle or at anterior half, with pair of discal depressions (sometimes reduced but visible, or, rarely, without depressions). Anterior pronotal border absent. Lateral margins rounded or subparallel in posterior half and rounded in anterior half, rarely explanate.
Elytra. Surface usually almost glabrous (with scattered erect setae on apical lobes only) or, rarely, densely covered with longer setae (the cervicornis -group). Elytra confusedly punctate and nonstriate, semistriate or slightly costate. Epipleura gradually narrowed to apex.
Legs. Procoxae globular, prosternal process reduced to thin depressed ridge, procoxal cavities closed. Protibiae usually simple, sometimes with emargination on inner side. Protarsomere I usually not modified, rarely almost circular (the insignis -group). Metatibia simple without apical spine or with subapical lobe sometimes with apical short black spine. Length of metatarsomere I about equal to following tarsomeres combined. Tarsal claws appendiculate with basal tooth small and rounded. Metatarsomere I usually simple, rarely slightly expanded and emarginated.
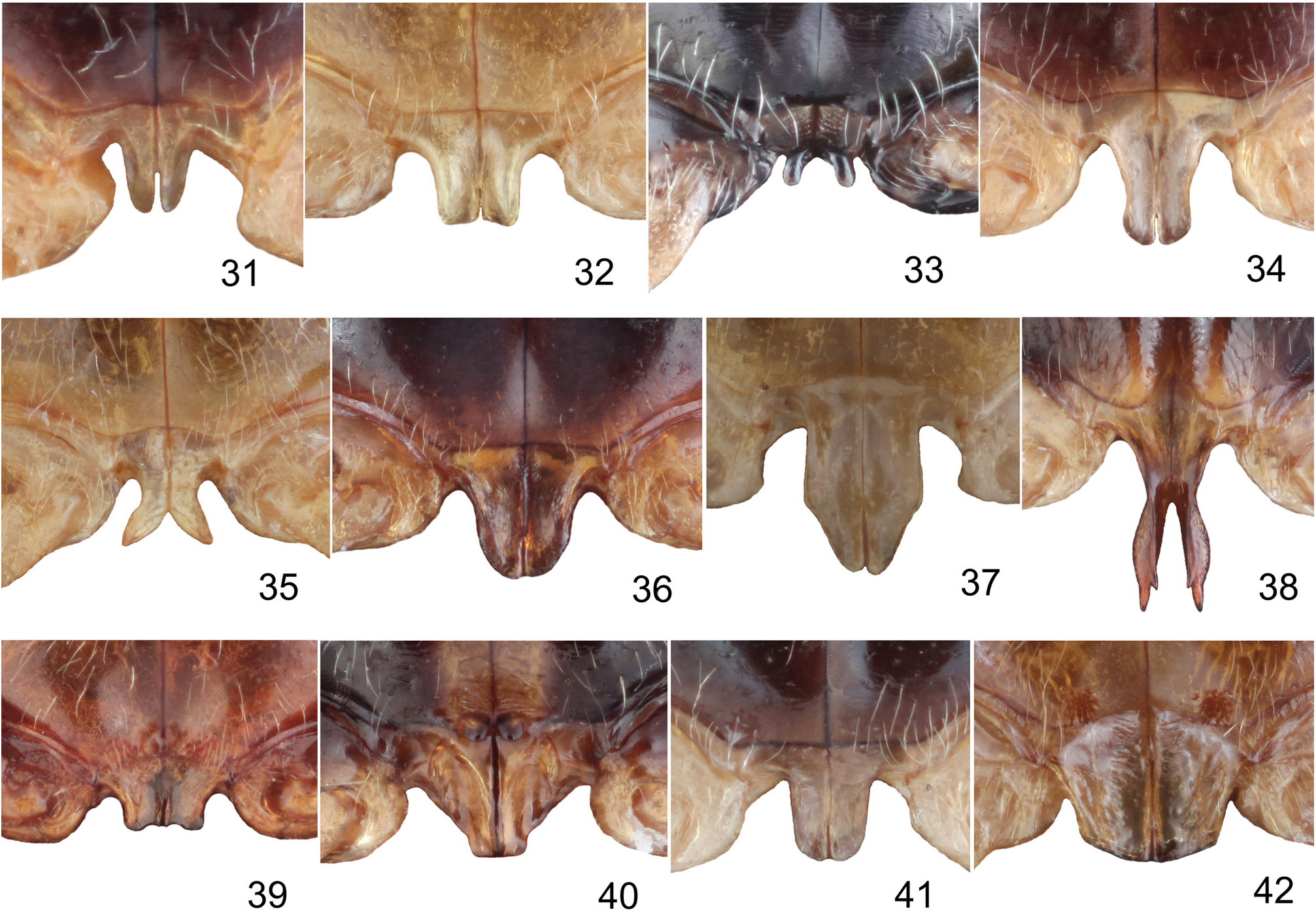
Metasternal process usually flat or slightly concave with thinly split apex, rarely apex bifurcate, divergent, whole process directed down, or accompanied with one or two pairs of small appendices ( Figs 31–42 View Figs 31–42 ).
Abdomen. Last ventrite apically trilobate.
Aedeagus always flatenned apically and with split apex ( Figs 55–63 View Figs 55–63 ). Ventral side with longitudinal groove (sometimes only in apical half), always distinctly narrowed subapically, constriction formed by two small triangular processes which can make distinct angulation in lateral view. In T. nasuta -group aedeagus dorsally with two small denticles near apex.
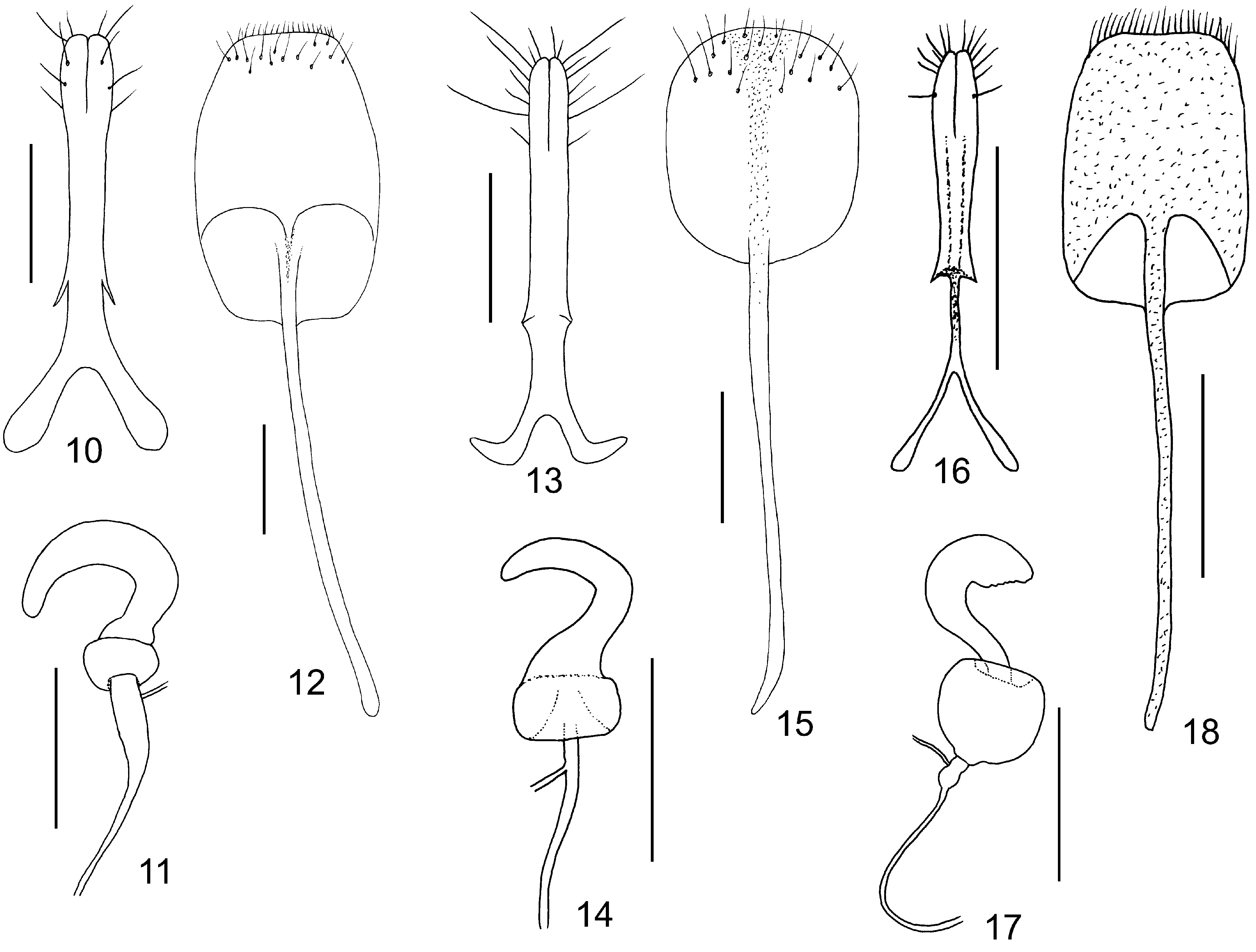
Females. Antennae slender, unmodified. Tibiae always without tibial spurs. Posterior margin of last ventrite regularly rounded, without incisions. Spermatheca with C–shaped cornu and well developed bulbus ( Fig. 11 View Figs 10–18 ). Gonocoxae with split apex, apical part with several long setae, base bifurcate ( Fig. 10 View Figs 10–18 ). Sternite VIII suboval, with setae cummulated in apical part, tignum thin circa 2–3 times as long as sternite VIII ( Fig. 10 View Figs 10–18 ).
Differential diagnosis. Taumacera is close to Palpoxena Baly, 1861 , and both genera can be treated as sister-genera ( REID 1999). They share the closed anterior coxal cavities, meso- and metatibia without an apical spine (some Taumacera have a spine on subapical lobe on metatibia), and a similar structure of the aedeagus. Female genitalia of Palpoxena and Taumacera are very similar, but they were studied only in a limited number of species.
Palpoxena itself is in need of revision. The characters presented here are valid for the Palpoxena laeta -group, in which the type species P. laeta Baly, 1861 ( Figs 2, 6–7 View Figs 1–9. 1–3 , 13–15 View Figs 10–18 ) belongs. The best character for separating the two genera seems to be the presence ( Taumacera ) or absence ( Palpoxena ) of a metasternal process in males. Males of Palpoxena always have filiform antennae, never with expanded or ridged antennomeres, while the antennomeres in males of Taumacera are often expanded, sometimes with a visible ridge (if slender, they are relatively robust, never filiform).Antennomere III is extremely long in Palpoxena , ca. 5.0–7.5 times as long as II and about 5.0–7.0 times as long as wide, while being more robust in Taumacera (ca. 3.5–6.0 time as long as II and about 2.5–5.0 times as long as wide). Palpoxena species have the frontal tubercles reduced to a very narrow, bent keel, and sometimes nearly absent, while the frontal tubercles in Taumacera are well developed. An excavated or modified frontoclypeus is typical for many Palpoxena males, while the head in Taumacera males is usually without such modifications (with some exceptions, e.g., the T. indica or the T. nasuta species-groups). Greatly expanded maxillary palps, previously used for separating both genera, are known only in the Palpoxena laeta -group, not in all Palpoxena species.
In past decades, Taumacera was often confused with Cerophysa . The differences between the two genera were already published by MOHAMEDSAID (1993) and REID (1999). Here, I expound on the differencies as follows ( Taumacera first): posterior margin of procoxal cavities closed (open in Cerophysa ); males with metasternal process (without process in Cerophysa ); pronotum more flat, with well-developed and visible lateral and posterior borders (pronotum more convex, with very thin lateral and posterior borders; lateral borders often not visible from above); unmodified antennomeres longer, ca. 2.5–5.0 times as long as wide (shorter, 0.7–2.0 times as long as wide in Cerophysa ); last visible ventrite with two deep narrow incisions on posterior margin (last visible ventrite with entire posterior margin in males of Cerophysa ); aedeagus flatenned apically, with longitudinal ventral groove distinctly narrowed subapically and with split apex (aedeagus extremely narrow, with apex bent in Cerophysa ). Female genitalia of Taumacera and Cerophysa are similar but should be studied on more extensive material. It is necessary to note that Cerophysa itself is also in need of revision as it is evidently comprised of several species-groups which should probably be classified in other genera ( BEZDĚK 2018). The abovementioned characters refer to the type species Cerophysa nodicornis (Wiedemann, 1823) from Java ( Figs 3, 8 View Figs 1–9. 1–3 – 9 View Figs 1–9. 1–3 , 16 View Figs 10–18 – 18 View Figs 10–18 ).
Clarifications of proposed synonymies. Taumacera males display great variability in the shape of the antennae and head which are, however, nothing more than secondary characters of sexual dimorphism. As shown by REID (1999, 2001) there is no justification for maintaining genera based only on a single secondary sexual character. The examples of the variability in males are shown in Figs 19–30 View Figs 19–30 for the antennae and in Figs 43–54 View Figs 43–54 for the head. REID (1999) proposed the T. deusta species-group for species with an expanded antennomere III. Additional six species-groups are established in the present paper. However, some species are not classified within any species-group for various reasons (unknown males, unclear identity etc.).
REID (1999) mistakenly classified Dorydea indica Jacoby, 1889 in the genus Palpoxena . Because Paraenidea azurea Laboissière, 1933 (the type species of Paraenidea ) was considered as a synonym of Dorydea indica, REID (1999) subsequently proposed Paraenidea to be a synonym of Palpoxena . Recently, MOHAMEDSAID & CONSTANT (2007) transferred Dorydea indica to Taumacera , but the generic synonymy was not solved in their paper. I studied the type specimens Dorydea indica as well as additional nontype specimens and the males have a well developed metasternal process ( Fig. 34 View Figs 31–42 ), therefore, I concur with MOHAMEDSAID & CONSTANT (2007) that Dorydea indica must be classified in Taumacera . Consequently, Paraenidea is removed from synonymy with Palpoxena and is newly synonymized with Taumacera .
MOHAMEDSAID (1997b) described the new genus and species Kinabalua antennata Mohamedsaid, 1997 from Sabah. The second species, K. musaamani , was added by MOHAMEDSAID (2010a). Kinabalua differs from Taumacera only in the structure of the antennae with dilated antennomeres VII and VIII, and a large sharp spine on antennomere VIII directed backwards ( Fig. 19 View Figs 19–30 , see also photographs in MOHAMEDSAID 2010a). This character is treated as a sound modification device ( MOHAMEDSAID 2010b). All other characters are shared with Taumacera including the presence of metasternal process. As the structure of male antennae is a secondary sexual character, I here propose Kinabalua to be treated as a new synonym of Taumacera .
The genus Xenarthra Baly, 1861 was proposed for Xenarthra cervicornis Baly, 1861 from Sri Lanka. An additional three species, also from Sri Lanka, were added by JACOBY (1887a). Males of all species from Sri Lanka have long lateral branches on male antennomeres III–X or on a part of these antennomeres ( Figs 20–21 View Figs 19–30 , 65–66, 68–69 View Figs 64–73 ). The type specimens of all four Xenarthra species were examined and the males have a metasternal process. As the structure of male antennae is a secondary sexual character, I here propose Xenarthra be treated as a new synonym of Taumacera .
The genus Acroxena Baly, 1879 differs from Taumacera only in the strongly modified head of males ( Figs 48–50 View Figs 43–54 ), which is also a secondary sexual character. All other important characters (the presence of a metasternal process, the structure of the antennae, pronotum and aedeagus, and metatibiae without an apical spine) are shared with Taumacera . The genus Neochrolea Jacoby, 1887 was never formally synonymized with Acroxena . However, the only species Neochrolea cavifrons Jacoby, 1887 , was synonymized with Aenidea facialis Baly, 1886 (now Acroxena ) by BRYANT (1923). Later on, MAULIK (1936) synonymized Neochrolea with Palpoxena which, with some doubts, was also followed by GRESSITT & KIMOTO (1963). I examined all relevant type material and in sum I remove Neochrolea from the synonymy with Palpoxena and propose both Acroxena and Neochrolea as new synonyms of Taumacera .
The genus Azlania Mohamedsaid, 1996 has a flat metasternal lobe but is not treated in this study. Azlania comprises four species from Malaysia and Indonesia with A. costatipennis ( Jacoby, 1896) as the type species. I examined the type specimens of A. costatipennis and they have a single apical spine on simple metatibiae, which separates Azlania from Taumacera and Palpoxena . The lack of additional material does not allow me to resolve the relation of Azlania to Taumacera . I can neither exclude future synonymy of both genera nor provide confirmation of its validity.
REID (1999) also speculated that Lasioxantha Kimoto, 1989 and Epaenidea Gressitt & Kimoto, 1963 might be junior synonyms of Taumacera , but due to insufficient descriptions he did not synonymize them formally. I examined paratypes of Lasioxantha fulva Kimoto, 1989 (the type species of Lasioxantha). The males do not have the metasternal process and the structure of aedeagus is also very different from that of Taumacera . Therefore, I can confirm Lasioxantha as a distinct genus. The validity of Epaenidea needs further study as I only had the opportunity to study female paratypes of Epaenidea subvirida Gressitt & Kimoto, 1963 (the type species of Epaenidea ). Epaenidea elegans Kimoto & Gressitt, 1966 from the Ryukyu Islands is surely not congeneric with Taumacera (male paratype examined). The third species, E. indochinensis Medvedev, 2004 , is here transferred to Taumacera based on study of the type material.
Definition of species-groups in Taumacera with updated check-list
The genus includes several species-groups defined predominantly by male antennal characters. Previously, only Taumacera deusta species-group was designated by REID (1999), six additional groups are proposed here. For the check-list and diagnosis of species groups see Table 1 View Table 1 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Taumacera Thunberg, 1814
| Bezděk, Jan 2019 |
Kinabalua
| MOHAMEDSAID M. S. 1997: 132 |
Paraenidea Laboissière, 1933: 66
| REID C. A. M. 1999: 3 |
| KIMOTO S. 1989: 202 |
| LABOISSIERE V. 1933: 66 |
Platyxanthoides Laboissière, 1933: 71
| KIMOTO S. 1989: 202 |
| GRESSITT J. L. & KIMOTO S. 1963: 685 |
| LABOISSIERE V. 1933: 71 |
Neochrolea
| MAULIK S. 1936: 580 |
| JACOBY M. 1887: 117 |
Metellus
| BRYANT G. E. 1923: 147 |
| WEISE J. 1913: 231 |
| JACOBY M. 1886: 63 |
Nacrea
| JACOBY M. 1891: 65 |
| BALY J. S. 1886: 29 |
Neocharis
| JACOBY M. 1881: 448 |
| SHARP D. 1877: 485 |
Acroxena
| BALY J. S. 1879: 462 |
Thaumacera
| GEMMINGER M. & HAROLD E. VON 1876: 3560 |
Dorydea: CHAPUIS (1875)
| CHAPUIS F. 1875: 245 |
Doridea
| REID C. A. M. 1999: 1 |
| WEISE J. 1922: 108 |
| BALY J. S. 1864: 236 |
Platyxantha
| REID C. A. M. 1999: 1 |
| BALY J. S. 1864: 233 |
Xenarthra
| BALY J. S. 1861: 298 |
Taumacera
| THUNBERG C. P. 1814: 48 |