Panulirus meripurpuratus
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4107.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:25B292ED-867B-4737-93CD-7C65102B69E0 |
|
DOI |
https://doi.org/10.5281/zenodo.6082105 |
|
persistent identifier |
https://treatment.plazi.org/id/0D1987BB-FF91-FFDC-FF75-FC54FC93F85C |
|
treatment provided by |
Plazi |
|
scientific name |
Panulirus meripurpuratus |
| status |
|
Panulirus meripurpuratus n. sp
( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 , 4 View FIGURE 4 B, 5)
Panulirus argus View in CoL .— Fonteles-Filho & Ivo 1980: 25 –32. — Ivo & Pereira 1996: 7 –94. — Melo 1999: 436, fig. 294. — Silva & Fonteles-Filho 2011: 19, 22–94. — Teschima et al. 2012: 5. — Faria-Junior et al. 2013: 29, fig 2. — Gaeta et al. 2015: 2, fig 4. Panulirus argus westonii View in CoL .— Sarver et al. 1998: 185. — Sarver et al. 2000: 871. — George 2006: 1289. — Chan 2010: 159. [Nomen nudum].
Type material. Holotype. Offshore area around Pau Amarelo Beach, Pernambuco (7°54'15.6"S, 034°49'14.3"W), 0 4 November 2015, sampled by traps (covo), obtained from local fisherman, male, CL = 178 mm, (Oceanographic Museum Petrônio Alves Coelho in Pernambuco—MOUFPE 15564).
Paratypes. 7 specimens obtained from local fisherman, sampled by traps (covo), offshore around Pau Amarelo beach Pernambuco (7°54'15.6"S, 034°49'14.3"W), 0 4 November 2015: 2 males (#3 CL = 92 mm, #5 CL = 112 mm) and 5 females (#1 CL = 72 mm, #2 CL = 132 mm, #4 CL = 78 mm, #6 CL = 81 mm, #7 CL = 87 mm) ( MOUFPE 15562).
Type-locality. Pernambuco state, northeastern coast of Brazil.
Etymology. From Latin Meri (plural of merus) + purpuratus (purple). Based on the main colour pattern in the meri of pereiopods that differentiates this species from the Caribbean P. argus .
Description of male holotype ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 B–E). Carapace 1.4 times longer than wide, slightly shorter than abdomen length; covered with sharp anteriorly directed spines; very large spines (horns) above orbits, laterally compressed, directed anterodorsally, posteriorly with small spines; 2 transverse rows of 4 gastric spines; cervical groove distinct, other regions demarcated by shallow groove, with almost imperceptible divisions between cardiac, branchial and intestinal regions ( Fig. 1 View FIGURE 1 ).
Abdomen smooth; somites 2–5 with shallow and incomplete transverse groove, without bristles, distinctly interrupted medially, especially on somites 3–4 ( Fig. 2 View FIGURE 2 E); maximum width of abdomen 4.8 times less than total body length. Male pleopods 2–5 leaf shaped, without endopods.
Telson and uropods hard proximally and membranous distally; hard part of exopod with line of 7 small spines distally; hard part of endopod with 3+1 spines distally.
Antennular plate broad, bearing 2 pairs of large spines arranged in a square, with 2 anterior larger spines in the limit of antennular plate and 2 smaller spines posteriorly in the middle of antennular plate ( Fig. 2 View FIGURE 2 B). Antennulae nearly 0.8 times total body length; outer flagellum setose distally and shorter and thicker than inner ( Fig. 1 View FIGURE 1 ). Distal article of antennular peduncle exceeding antennal peduncle and over reaching first pereiopods ( Figs. 1 View FIGURE 1 ); second and third articles equal in length, half length of first article. Antennae very large, heavy, exceeding body length; peduncle reaching the distal limit of second antennular peduncle article; peduncle with numerous strong spines; ventral surface of first article smooth, with only 1 distomedial spine; flagellum stout, stiff, line of setae along inner margin and ringed with spines at intervals ( Fig. 1 View FIGURE 1 ).
Third maxilliped with exopod ( Figs. 2 View FIGURE 2 D); reaching carpi of first pereiopod; exopod with multi-articulate flagellum reaching 2/3 length of merus of endopod; first segment of exopod half length of flagellum ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 D). Exopod of second maxilliped with multi-articulate flagellum reaching beyond endopod; first segment of exopod slightly shorter than flagellum ( Fig. 2 View FIGURE 2 C).
Pereiopods unequal in size; pereiopod 2 longest, followed by pereiopods 3, 4, 5 and 1; dactylus about half length of propodus, covered with bristles; merus with dorsodistal and outer distoventral spines; carpi with longitudinal dorsolateral grooves. Pereiopods 3–5 ending with superior spines. Pereiopod 1 slightly exceeding the end of antennal peduncle, reaching the end of second antennular article and reaching 1/3 length of propodus of pereiopod 2. Pereiopod 1 little longer than carapace length; propodus with ventral bristles. Pereiopod 2 about 1.4 times longer than carapace.
Colour of male holotype ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 E). Antennal flagella purple. Mosaic of small purple dashes on antennal peduncle, antennular plate and anterolateral surface of carapace. Antennules orange-greenish. Supraorbital horns and ocular peduncle black and white. Body and pereiopods colourful dorsally, with inferior part whitish with reticulated sparse dashes.
Carapace with light red gastric region; base of spines dark red; cardiac region red with darker red tones on last four rounded spines; intestinal region red near cardiac region, with four darker red rounded spines on each side; branchial region with gradient of red, darker only posterodorsally near cardiac region (lighter anteriorly) and lighter laterally, with only rounded spines with darker colours; posterior margin of carapace with dashed line of dark red rounded spines; very light mosaic of granules laterally and posteriorly in carapace.
Meri of pereiopods purple without lines; carpi, propodus and dactylus with predominant orange yellowish colour; longitudinal purple line only in the grooves on each side of carpi; propodus without lines.
Abdomen light reddish brown; line of very small white spots (not continuous line) along posterior region of each abdominal somite; grooves at somites 2–5 posteriorly lined by very small white spots; abdominal somite 2 with two large, irregular, white eyespots with black margin, one on each lateral, and with two irregular whites spots centrally; abdominal somite 6 also with two large, irregular, white eyespots with black margin, one on each lateral and further areas irregularly white spotted; abdominal somites 3–5 with line of four small white eyespots with black margin dorsally and further areas irregularly white spotted.
Membranous part of tail fan (telson and uropods) greenish with two black transverse bands distally and proximally, and brownish band centrally. Protopod and hard part of uropods and telson with same colour pattern as abdomen but with larger white dashes. Pleopods black medially with yellow-greenish margin.
Variation in Paratypes. In some specimens the antennular plates presented 1–4 additional spines between the main four spines. Paratype males present the same morphological proportions as the holotype. In one male specimen, the antennae are 1.7 times the total length of body.
In females, the third antennular article does exceed the antennal peduncle and still proximally 0.8 times the total length of body. The first pereiopod is shorter and in most specimens does not reach the end of the antennal peduncle. The second pereiopod is proportionally shorter than in males at 1.02–1.07 times carapace length. The fifth pereiopod of females is distally subchelate. The antennae are 1.5–1.7 times the total length of body (few specimens with complete antennae for comparisons). The exopod of maxilliped 2 is almost same size as flagellum or 0.8 shorter. The carapace is 1.6–1.8 times longer than wide and 0.8–0.9 times shorter than abdomen length; maximum width of the abdomen is 3.8–4.3 times less than the total body length. The female pleopods on the abdominal somite 2 have a leaf-shaped endopod similar to exopod but slightly smaller; pleopods 3–5 with bifid endopods ( Fig. 2 View FIGURE 2 G, H).
Thus, sexual dimorphism is evident in reproductive males, which present a larger cephalothorax, longer antennular peduncle and longer pereiopod 2 used in mating. Reproductive females have a larger abdomen and laminar endopods of pleopod 2; abdominal somites 3–5 with bifurcated endopods modified for carrying eggs; and the fifth pereiopod with a sub-chela for grooming.
Colour variation was not related to sexual dimorphism. Some adult males and females present a paler carapace and abdomen ( Fig. 2 View FIGURE 2 A); as well as more purplish than red on the dorsal regions and more greenish antennules and abdomen ( Fig. 2 View FIGURE 2 A). The spotted line on the transverse groove of the abdominal somites is sometimes merged, but the grooves remain separated ( Fig. 2 View FIGURE 2 F).
Speciation. The differentiation between P. argus from the Caribbean sea and P. meripurpuratus from Brazil, according to genetic analysis occurred between 8.4 and 26.1 My ( George 2006; Sarver et al. 1998, 2000; Tourinho et al. 2012). During this period at the beginning of late Miocene (around 10 My) with the Last Glacial Maximum, a great change took place in the western Atlantic Ocean with the beginning of the outflow of the Amazon-Orinoco River ( Campbell Jr et al. 2006). This created a massive physical barrier of low salinity water isolating biological populations and enabling allopatric speciation and vicariance. Indeed, the Amazon-Orinoco River plume is currently the most prominent biogeographic barrier in the western Atlantic Ocean, separating two tropical ecoregions with great differences in biodiversity composition ( Spalding et al. 2007; Floeter et al. 2008; Rocha et al. 2008). This biogeographic barrier is reported as the main factor leading to allopatric speciation ( Sarver et al. 1998, 2000; Tourinho et al. 2012) separating P. a rgu s and P. meripurpuratus into genetically different populations. Isolating P. argus to the region north of the Amazon river plume and P. meripurpuratus to the south in Brazilian waters.
Distribution. From Pará (2°S) to Santa Catarina (27°S) on the Brazilian coast, including the oceanic island of São Pedro and the São Paulo Archipelago, Fernando de Noronha and Rocas Atoll ( Melo 1999; Silva & Fonteles- Filho 2011; Teschima et al. 2012; Gaeta et al. 2015). Freitas & Castro (2005) reported P. a rgu s (but with the characteristics of P. meripurpuratus ) from Cape Verde; however Tourinho et al. (2012) reported an uncertainty about these populations and suggested that the species presence was instead due to anthropogenic transport. Sarver et al. (2000) described the presence of P. meripurpuratus (as the Brazilian form of P. a rgu s) in Caribbean Waters was also uncertain of the provenance of local populations.
Biology. (Based on Ivo & Pereira 1996; George 2006; Silva & Fonteles-Filho 2011; Butler et al. 2013; Giraldes et al. 2015a; b; as P. argus in Brazil). Panulirus meripurpuratus presents two distinct migration behaviours: the trophic migration in the first three or four months of the year, with random displacement parallel to the coast searching for sites with high food concentration; and the reproductive (ontogeny) migration trough deeper areas in the first and second trimesters searching for favourable sites for reproduction. Like other lobsters reproduction occurs by front position mating, with male depositing the sperm mass on the sternum of the female. Fertilized eggs are attached to the exopod of pleopods of the female. Planktonic larvae stay for 12 months in the water mass where they assume a benthic position and become a puerulus; in the next 12 month the puerulus stage remains in shallow water for 1–2 years of life during which it becomes a juvenile; the juveniles stay in coastal reefs for 3 years during which they become adults in a pre-reproductive stage and migrate to deeper water for reproduction. This species presents a long life cycle of approximately 18.5 years. Adults are found in reproductive condition every month in tropical regions; however, the greatest reproductive intensity occurs from January to April. The average length of females at the first stage of sexual maturity is estimated at 20.5 cm in total length (7.5 cm for carapace length and 13.0 cm for tail length). The species presents a high fecundity with an average of 29,4175 eggs per female and 630 eggs /g of adult female; larger females produce more eggs and also incubate a greater number of eggs than smaller females. The sex ratio in the adult population supposedly is higher for males due to a numerical predominance of captured males reported in fishing stock description. The diet consists mainly of molluscs and crustaceans and as secondary food, echinoderms, algae and corals. Feeding habits are based on nightly foraging. The recruitment is dependent on the number and size of females. The natural recruitment ratio of new specimens into the population for this species in Brazil is uncertain, as biological data has traditionally been provided by analysis conducted by the fishing industry. It is important to report that the recruitment of this species happens in shallow coastal reefs and as suggested by Giraldes et al. (2012, 2015a, b), it is possible to monitor this large lobster in coastal areas using nocturnal underwater visual cense and obtain information about species recruitment using low impact methods.
Habitat. (Based in Ivo & Pereira 1996; Rocha et al. 1997; Melo 1999; George 2006; Silva & Fonteles-Filho 2011; Giraldes et al. 2012, 2015a; b; as P. argus in Brazil).
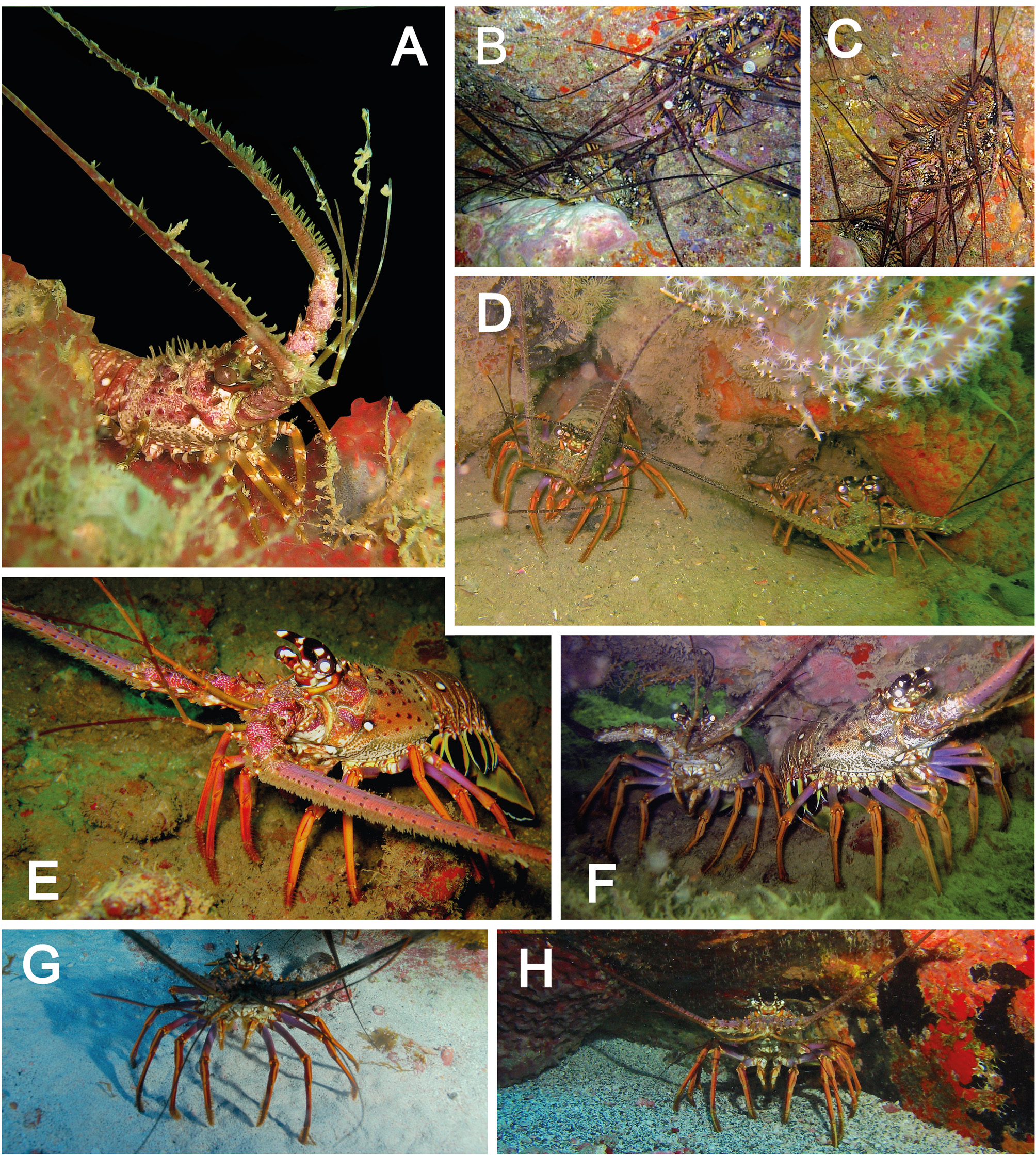
Panulirus meripurpuratus is usually found on reefs and coralline algae, sponges or other objects which afford protection or places of concealment; from low-tide mark to depths of about 50 m; increasing the abundance perpendicular to coast, with a maximum abundance at around 41– 50 m. There are different habitats according to the life stage. Post larvae, puerulus and early stage juveniles live on outer reef habitats in coastal shallow reefs, associated with sessile benthic organisms and fouling, such as algae ( Fig. 3 View FIGURE 3 A). Juveniles inhabit shallow coastal reefs (around 0–15 meters) but small juveniles are found on the roof of caves inside cavities ( Figs. 3 View FIGURE 3 B, C) and large juveniles (or young adults in a pre-reproductive stage just before the ontogenic migration) are found in cavities at the interface between the soft bottom and the reef/rock; at this time it is usually observed at night walking on the bottom near the reef structure ( Figs. 3 View FIGURE 3 D–F). Adults are found in deeper water around 20–50 m after the reproductive migration; observed by day and at night usually in soft bottom near a sheltered area ( Figs. 3 View FIGURE 3 G, H). During migration, it is found in groups on soft bottom habitats walking through deeper reefs. The main description for P. meripurpuratus associated habitat is based on reports from commercial fishing activity and by nocturnal scuba diving observations.
Fishing history. (Based on Ivo & Pereira 1996; Rocha et al. 1997; Silva & Fonteles-Filho 2011; Giraldes et al. 2015b; as P. argus in Brazil).
The fishing of P. meripurpuratus as a target species in Brazil started around 1950, with the aim to export frozen lobster tails as they commanded a high market price. In Brazil, the species was fished intensely from Pará to Espírito Santo state an area of around 74.607 km 2. The fishing methodologies centre around three types of techniques: traps (covo or cangalha), anchored bottom gillnets (caçoeira) and diving (free diving or compressor diving); of the three fishing methods the trap is presently the only legalized technique nowadays. The Northern sub-region comprises the coast of the states of Amapá, Pará and Maranhão and despite the large marine area the population of this species presents a low density due to the strong influence of fresh water plumes from the Amazon and Orinoco rivers. This has caused a reduction in the fishing catchment area, which has pushed lobster fishing activities into deep-water sites offshore. The large continental shelf area of the Northern Northeast subregion from Piauí to Rio Grande do Norte state, has a habitat of calcareous algae and rocks and delivers the highest recorded landings for this species in Brazil. The East Northeast sub-region, from Rio Grande do Norte to Espírito Santo state supports a large number of coralline reefs and a high abundance and diversity of demersal fish of economic value to the fishing industry. This region has the lowest catch per unit effort within the commercial lobster fishing industry and the lowest recorded landings of lobsters of the three sub-regions. There is no commercial fishing activity between Espírito Santo and Santa Catarina State. Historically the most intense fishing activity takes place between the Northern Northeast and East Northeast sub-regions, with Ceará and Pernambuco state accounting for about 80% of the total catch for the export market. Since the 1960s the region has recorded a drastic reduction in landings for this species. In the past advances in fishing technology have shown false increases in population numbers with increases in catch not a true representation of a standing stock but attributed to improvements in fishing methodologies. A pattern in increased landings can also be seen when the market value of a target species increases fishing effort also increases leading in-turn to increased landings. This can sometimes be wrongly interpreted as a recovery in stock. Unfortunately due to the constant increase in the fishing effort and the increase in size of the fishing fleet the stock abundance of this species is in a state of constant decline. Clearly the management and oversight strategies to control and limit the capture of this species by the Brazilian government have not been rigorous enough to address the overfishing scenario. A major concern about the management of this species was highlighted by Giraldes et al. (2015b) when particular emphasis was focused on the importance of protecting the natural nursery regions of the shallow coastal reef ecosystem (<15m).
Remarks. Panulirus argus (Latreille, 1804) remains the name for the species indigenous to the Caribbean Sea. The original description by Latreille (1084: 393 as Palinurus argus ) is very limited and could apply to both P. argus and P. meripurpuratus . Images, pictures and descriptions for P. argus reported in references such as Crawford & Smidt (1922: 291, figs. 265–271), Williams (1984: 170, fig. 120), Williams (1986: fig. 44, fig. 79b–c), Holthuis (1991: fig. 249, 257) and Cervigón et al. (1992: 143 pl. II, fig 13) are based mainly on specimens from regions north of the Amazon-Orinoco river barrier. It was on examination of these references that differences between the specimens of P. meripurpuratus and P. argus were first discovered.
The characteristics used to differentiate P. a rgu s and P. meripurpuratus have been used as part of the key identification presented below. P. argus presents carapace with deep grooves well defining all regions, with very distinct divisions among cardiac, branchial and intestinal regions; while the carapace in P. meripurpuratus presents only distinct cervical groove, with shallow groove dividing the regions, faintly perceptible divisions between cardiac, branchial and intestinal regions. Abdominal somites 2–5 in P. a rgu s with deep transverse groove often faint medially (not distinctly interrupted); and in P. meripurpuratus abdominal somites 2–5 present shallow and incomplete transverse groove, interrupted medially, especially on somite 3. The conspicuous spots are observed in both species but they are isolated in the abdomen of P. a rgu s presenting only the scattered spots; while in the abdomen of P. meripurpuratus the conspicuous scattered spots are mixed with several small spots. The abdomen is darker, with solid colour in P. argus and in P. meripuratus it is lighter and spotted. In the grooves and at the end of each abdominal somite, P. argus present continuous light line and P. meripurpuratus present small white spots in line. Carapace of P. a rgu s has an intense and solid dark red colour dorsally, extending onto lateral surfaces; while the carapace of P. meripurpuratus has a light background with pale red (sometimes almost pink), with darker areas mainly in cardiac region and on dorso-posterior area of branchial region (near the cardiac) and only the base of spines are dark red, forming conspicuous dark spots in the light background. Pereiopods are striped longitudinally in P. argus , including meri and propodus; and in P. meripuratus the pereiopods are mostly without stripes (present only on carpi), with peculiar conspicuous and solid purple colour on meri, with orange/brown shades at propodus and dactylus. Pleopods in P. argus are yellow-green with a longitudinal black line medially with sickle shape; in P. meripuratus it pleopods present only a lateral border green or yellowish with a large black area in centre (not only a black sickle shape line).
The distribution of Panulirus argus (Latreille, 1804) is now restricted to northern localities from North Carolina (35°N) to Venezuela (8°N) including the Caribbean Sea, West Indies, Gulf of Mexico and Bermudas—localities north of the biogeographic barrier of the Amazon River plume ( Williams, 1986; Holthuis, 1991; Sarver et al. 2000; Tourinho et al. 2012). At localities south of the Amazon River plume on the Brazilian marine domain (from 2°S to 27°S) P. meripurpuratus is the representative species (see distribution section above). It was highlighted that neither P. argus nor P. meripurpuratus were reported between the parallels of 8°N and 2°S, the area which is influenced by the Amazon-Orinoco River ( Sarver et al. 2000; Tourinho et al. 2012). It can therefore be regarded as a physical barrier blocking the gene flow and isolating the two populations. Sarver et al. (2000) reported that there was no recorded specimen of the Caribbean P. a rgu s in Brazilian waters and that the presence of P. meripurpuratus upward of the biogeographic barrier is uncertain. However, after the formal description of P. meripurpuratus presented in this study, it is now be possible to confirm whether the two species populations remain in isolation or co-habit within a specific region. It should also be noted that the number of Atlantic Ocean Panulirus species has increased to six species.
| MOUFPE |
Oceanographic Museum of the Federal University of Pernambuco |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Panulirus meripurpuratus
| Giraldes, Bruno Welter & Smyth, David Mark 2016 |
Panulirus argus
| Teschima 2012: 5 |
| Silva 2011: 19 |
| Chan 2010: 159 |
| George 2006: 1289 |
| Sarver 2000: 871 |
| Melo 1999: 436 |
| Sarver 1998: 185 |
| Ivo 1996: 7 |
| Fonteles-Filho 1980: 25 |