Limnonectes megastomias, S, David, 2008
|
publication ID |
https://doi.org/10.5281/zenodo.182721 |
|
DOI |
https://doi.org/10.5281/zenodo.5675639 |
|
persistent identifier |
https://treatment.plazi.org/id/0D1C8787-0307-DE07-FF3E-FE43AFD2D19F |
|
treatment provided by |
Plazi |
|
scientific name |
Limnonectes megastomias |
| status |
sp. nov. |
Limnonectes megastomias View in CoL , new species
Holotype CUMZA 2003.134 deposited at CUMZ, adult male, collected from spring-fed pool at head of intermittent stream in deciduous evergreen forest at Sakaerat Environmental Research Station, Nakhon Ratchasima Province, Wang Nam Khieo District, Thailand, 14°29.680'N 101°52.257'E, 645 m, on 27 June 2003 at 1900 hr by DSM and Taksin Artchawakom.
Paratopotypes. Total of 15 specimens: 11 males, 4 females. CUMZA 2003.135 (female) collected with holotype ( Fig. 3 View FIGURE 3 B); KU 307760–63, 307766–72 (males), KU 307764–65, 307773 (females), KU 307776 (male, dry skeleton), KU 307774 (male, cleared-and-stained), KU 307777 (female, dry skeleton), KU 307775 (female, cleared-and-stained), collected at same locality as holotype on 15 March 2005 at 1840–2130 hr by DSM, Kyle Hesed and Taksin Artchawakom.
Paratypes. Total of 20 specimens: 14 males, 6 females. FMNH 266212, 266219 (females); FMNH 266213–218 (males), collected in Phu Luang Wildlife Sanctuary, Loei Province, Phu Rua District, Thailand, 17°16.812' N 101° 31.125'E, 1460 m, on 2–3 September 2004 by Yodchaiy Chuaynkern, Bryan L. Stuart, Chatchay Chuechat, and Sunchai Makchai. FMNH 266220, 266223, 266229, 266231 (females); 266221–22, 266224–27, 266230, 266232 (males); 266228 (juvenile), collected in Pang Si Da National Park, Sa Kaeo Province, Sa Kaeo District, Thailand, 14°6.345'N 102°15.693'E, 600 m, on 21, 26–27 September 2004 by Yodchaiy Chuaynkern, Bryan L. Stuart, Chatchay Chuechat, and Sunchai Makchai.
Other meaterial. FMNH 266340 tadpoles collected in Pang Si Da National Park, Sa Kaeo Province, Sa Kaeo District, Thailand, 14° 6.345'N 102° 15.693'E, 600 m, on 21 September 2004 by Yodchaiy Chuaynkern, Bryan L. Stuart, Chatchay Chuechat, and Sunchai Makchai. KU 307778–307784 tadpoles and eggs (hatched and reared in field lab) collected at type locality on 15 March 2005 at 1430 hr by DSM, Kyle Hesed and Taksin Artchawakom.
Diagnosis. Limnonectes megastomias , a large species of Limnonectes allied to the kuhlii species group as characterized by the following combination of characters: (1) adult male SVL 40.0– 123.7 mm (mean = 80.2; SD ± 23.66; n = 26), adult female SVL 53.5–86.3 mm (mean = 74.0; SD ± 11.29; n = 10); (2) tympanum obscured by thickened skin; (3) vocal sac and vocal slits absent in males; (4) males have advertisement call; (5) males with nuptial pads on Fingers I and II; (6) males with thick, elongate odontoid processes; females with reduced odontoid processes; (7) males with enlarged heads (HL 41–56% of SVL; 39–45% in females); (8) prominent supratympanic fold from posterior superior corner of eye to angle of jaw; (9) throat, venter, and borders of thigh and leg heavily pigmented (mottled) in males, females with lightly pigmented throat, moderate pigmentation on venter and borders of thigh and leg; (10) small, low glandular warts tipped with translucent horny spinules on flanks of body, around vent, and on dorsum of shank and foot; dorsal skin moderately rugose except for regions covered with warts, ventral skin smooth; (11) Finger II longer than Finger I when adpressed; (12) no webbing between fingers, toes fully webbed ( I 0–0+ II 0–0+ III 0+–0 IV 0–0 V); (13) larval labial tooth row formula: 2(2)/3(1); (14) narrow gap in ventral oral papillae of larvae; (15) eggs pigmented; eggs deposited in large numbers with no parental care.
Comparisons. As noted by Emerson et al. (2000), frogs of the genus Limnonectes are phenotypically similar. Whereas the most distinguishing feature of Limnonectes megastomias is its tremendous maximum size (SVL) when compared to other species allied to L. kuhlii , reliance on external morphological characters alone for species determination is inadequate for this group. Based on the results of the molecular phylogenetic analysis placing the new species within Limnonectes , and because of the genetic distinctiveness of Limnonectes megastomias , I have elected to limit morphological comparisons of the new species to those species with which it is (1) most closely related, (2) geographically proximate to, and (3) with those it would most easily be confused: Limnonectes kuhlii (from the type locality, Java, and specimens currently recognized as L. kuhlii from Thailand and Laos), the phenotypically similar Limnonectes laticeps Boulenger (1882) , and the large-bodied Limnonectes namiyei Stejneger (1901) . Emerson and Berrigan’s (1993) clade consisting of L. kuhlii , L. namiyei , and L. laticeps is united by six synapomorphies, three of which are considered here (an obscured tympanum, short vomerine tooth row, and the presence of nuptial pads), features also shared by L. megastomias .
Limnonectes namiyei View in CoL is endemic to Okinawajima Island in Japan ( Maeda & Matsui, 1990), and is considered by Emerson and Berrigan (1993) to be the sister taxon to L. kuhlii View in CoL . Limnonectes megastomias View in CoL can be easily distinguished from Limnonectes namiyei View in CoL by the presence of vocal slits in male L. namiyei View in CoL (absent in L. megastomias View in CoL ). Limnonectes megastomias View in CoL is much larger, with a SVL of 123.4 mm, than L. laticeps View in CoL . Berry (1975), Boulenger (1912), and Chan-ard (2003) reported a maximum SVL = 53 mm for L. laticeps View in CoL . Limnonectes megastomias View in CoL has more extensive toe webbing than L. laticeps View in CoL (%–¾ webbed based on Boulenger (1920), I 1–2 II 1–2 III 1–3 - IV 3 -– 1 V in examined specimen of L. laticeps View in CoL ; webbing formula = I 0–0+ II 0– 0+ III 0+–0 IV 0–0 V in L. megastomias View in CoL ), and nuptial pads on Fingers I and II (only on Finger I in L. laticeps View in CoL ). Limnonectes megastomias View in CoL can be distinguished from the L. kuhlii View in CoL from the type locality of Java, and other recognized geographic forms of L. kuhlii View in CoL on the basis of maximum SVL (Table 1). Limnonectes megastomias View in CoL differs from Javan ( type) L. kuhlii View in CoL in having Finger II longer than Finger I (I> II in Javan L. kuhlii View in CoL ). Based on specimens examined, it seems that males of L. kuhlii View in CoL from Java lack nuptial pads (see discussion), whereas males of L. megastomias View in CoL have well-developed nuptial pads.
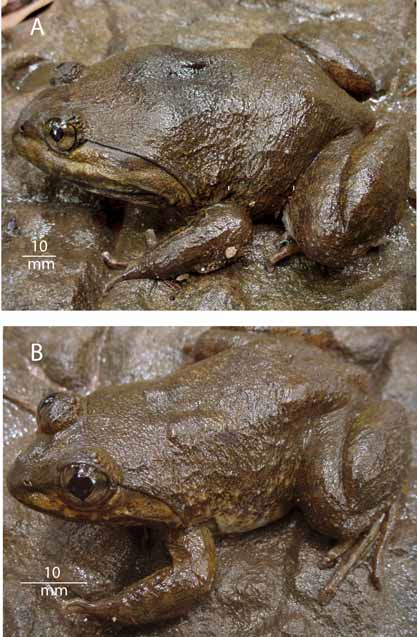
Description of holotype. Adult male ( Figs. 3 View FIGURE 3 a and 4). Habitus robust with greatly enlarged head (HL = 56% of SVL); head longer than wide (HW = 92% of HL). Rostrum rounded in dorsal view, projecting beyond lower jaw, obtuse (sloping) in profile; nostril dorsolaterally oriented, closer to tip of snout than eye; internarial distance 74% of interorbital distance; canthus rounded; lores concave; upper lip distinctly swollen and flared, extending to post-rictal tubercle; eye diameter 15% head length; width of upper eyelid 63% of interorbital distance; pupil diamond shaped. Supratympanic fold moderate, extending from eye to angle of jaw (insertion of arm); tympanum not visible. Vomerine teeth on oblique ridges, separated from each other by width of one ridge. Choanae oval, perpendicular to the longitudinal axis of the body. Odontoid processes robust, more than twice the depth of the mandible at base of processes. Symphysial knob at mandibular symphysis. Tongue oval, deeply notched posteriorly. Tips of all four fingers rounded, not expanded into discs but with rounded distal pad; relative lengths of fingers: 3421; no webbing between fingers; distinct, movable fringe of skin on pre- and postaxial sides of Fingers II and III, indistinct fringes of skin on Finger IV; digits indicated by roman numeral (tubercle count in parentheses): IV (1), III (2) II (1), I (1); proximal subarticular tubercles prominent, round, elevated, bifid on Fingers II and III ( Fig. 4 View FIGURE 4 A); distal subarticular tubercles low, flat and indistinct, bifid on Finger III; thenar metacarpal tubercle large, oval, not elevated; inner metacarpal tubercle oval, smaller than thenar tubercle, not contacting outer or thenar tubercles; outer metacarpal tubercle smaller than inner tubercle, oval, elevated; prominent nuptial pads composed of minute spines on medial surface of Finger I and dorsomedial surface of Finger II above preaxial fringe. Tips of toes rounded, not expanded into discs, toe pads elevated; relative lengths of toes: 43521; toes webbed to middle of terminal phalanx (webbing formula = I 0– 0+ II 0–0+ III 0+–0 IV 0–0 V); distinct, movable flap of skin on postaxial side of Toe V from middle of terminal phalanx to proximal end of metatarsus; distinct, movable flap of skin on preaxial side of Toe I from middle of terminal phalanx to level of inner metatarsal tubercle, continuing as weak fold on distal one third of tarsus; subarticular tubercles prominent, elevated, round; digits indicated by roman numeral (tubercle count in parentheses): V (2), IV (3) III (2), II (1), I (1); inner metatarsal tubercle oval, elongate with elevated post axial bor- der. Skin on top of head, dorsal surface of limbs, and dorsum moderately rugose; skin on sides, dorsum of lower arm, around vent, dorsum of shank and foot distinctly rugose, covered with small, low glandular warts with pearl tips; ventral skin smooth; weak, transverse fold between posterior margins of eyes.
Measurements. Morphometric data for the holotype (male, CUMZA 2003.134) are: SVL = 121.9; ED = 10.2; EN = 10.7; RL = 19.7; FEL = 58.6; FOL = 68.2; HL = 68.1; HW = 62.7; IN = 8.9; IO = 12.0; LAL = 23.2; MN = 57.6; PAL = 27.3; TBL = 48.3; UEW = 7.6; OH = 9.6; MH = 4.5.
Color of holotype in life. Based on color images shown in Figure 3 View FIGURE 3 A. Dorsum olive-brown with dark brown blotch over shoulders and indistinct, dark transverse bars on upper surface of hind limbs; side of head and lateral surfaces of body yellowish brown; chin brownish white; belly white with brown vermiform markings; dark brown bar between eyes bordered by thin yellowish-brown bars; lower half of iris gold, upper half brown, separated by dark brown horizontal band; nuptial pad white.
Color of holotype in perservative. Similar to color in life. Areas colored yellowish brown in life appear gray brown in preservative; white coloration on venter appears gray.
Description of Tadpole. The following description is based on an individual at Gosner (1960) Stage 40 (FMNH 266340). The tadpole is illustrated in Figures 5 View FIGURE 5 A and B. Body oval, slightly depressed, with midbody height being about 24% of body length; tail slightly higher than body, dorsal fin arising slightly behind origin of caudal musculature, maximum height just posterior to mid-length, tapering gradually until near tip where it narrows abruptly to narrow, rounded tip; tail about 2 times body length. Maximum body width 59% of body length; body height 24% of body length. Eyes large (approximately 12% of body length; 50% of body height at level of eyes); positioned dorsolaterally, oriented laterally; interorbital distance 41% of body width. Nares between tip of snout and eyes, rim not raised; internarial distance 61% of interorbital distance. Spiracle closer to eye than to end of body, midway up side, end of tube free of body wall.
Oral disk ventral, near anterior end of body; posterior labium with single, staggered row of short, thick papillae, with narrow median interruption; papillae of anterior labium in single row, confined to corners; labial tooth formula 2(2)/3(1), outer posterior row much shorter than other rows; jaw sheaths marginally black, serrate, anterior jaw sheath with weak median convexity.
Color of Tadpole in life. ( Fig. 5 View FIGURE 5 C) Body brownish gold with faint dark brown mottling on dorsum, without pigment on venter; iris gold with median dark brown horizontal bar; caudal muscle and dorsal fin brownish gold with faint dark brown mottling and distinct spots over entire length; ventral fin without brown pigmentation in proximal half, mottled and spotted in distal half.
Color of Tadpole in preservative. Similar to color in life. Areas colored brownish gold in life appear yellowish white in preservative.
Measurement of Tadpole. Morphometric data for the Stage 40 individual (FMNH 266340) are: BL = 14.4; BW = 8.5; BH = 3.5; ToL = 42.4; LED = 1.8; LIO = 3.5; LIN = 2.2; LNS = 1.8; LEN = 1.4; SW = 0.8; TMH1 = 3.5; TMH2 = 3.3; TH = 7.0; TL = 28.0
Variation. Measurements of paratypes and congeners are summarized in Table 1. Most striking is the enlargement of the head in males of Limnonectes megastomias . The progressive, disproportionate, enlargement of the head in males is well documented in Limnonectes kuhlii ( Hiroshi & Matsui, 2002; Inger, 1966; Pope, 1931), and is associated with male-biased sexual dimorphism and male-male combat behaviors ( Hiroshi & Matsui, 2002). Though no male-male combat behavior was observed in L. megastomias , specimens were collected at SERS that had missing digits and limbs, fresh bite marks, and scars indicative of either predation attempts or conspecific combat. Additionally, in L. megastomias , when head length is subtracted from SVL, adult males have smaller bodies (53% SVL) than adult females (58% SVL), a trend also seen in populations of L. kuhlii ( Pope, 1931) . Curiously, there is a great deal of variation in the SVL of males with nuptial pads ( 40– 123.7 mm), which also may be related to sexual selection and competitive male-male interactions. Hiroshi & Matsui (2002) reported that larger males are more successful in male-male combat, and suggested that males defending suitable oviposition sites are more likely to have access to females, and as a result, mate more frequently. At SERS, very large males (i.e., SVL> 90 mm) were encountered less frequently, were more wary and likely to seek cover in mud and debris when disturbed, and thus, were more difficult to collect than smaller males (i.e., SVL < 70 mm).
Most specimens are uniformly brown in dorsal coloration and lack dark-colored bars on the forearm and hind limbs. A few specimens from each population have a lighter dorsal coloration and have distinct bars on the forearms and hind limbs. The holotype, and some (23 of 36) of the syntypes have bifid subarticular tubercles ( Fig. 4 View FIGURE 4 ). This character occasionally varies from side-to-side in the same animal. In the SERS population, all specimens examined (16 individuals) have bifid subarticular tubercles, most frequently the proximal subarticular tubercles on Fingers II and III, but occasionally distal tubercles on the same fingers. In the Pang Si Da population, eight of thirteen individuals have bifid subarticular tubercles, most frequently the distal tubercle on Finger III (6 of 8). None of the individuals from the Pu Luang population examined has bifid subarticular tubercles. Additional variation is seen in the degree to which the tympanic annulus was visible. In 12 of 37 specimens of L. megastomias , the tympanic annulus was sufficiently visible (though always partially occluded by the supra tympanic fold) to be measured, though it is unclear whether this is an artifact of preservation, or a variable character within this species (see discussion below).
Morphological variation observed in a series of 14 tadpoles is summarized in Table 3 View TABLE 3 . The largest total length of 44.0 mm was observed in a Stage-41 tadpole, the smallest (28.0 mm) in a Stage-28 tadpole. During development, the relative difference between internarial and interorbital distances increases (LIN/LIO = 70% at Stage 28 and 46–51% at Stage 41). Though there is variation in individual measurements, none seems to be noteworthy.
Etymology. The specific name megastomias is a combination of the Greek words mega, meaning "large" and stomias, which is the masculine noun for "large" or "hard-mouthed" animal. The most striking characteristics of the males of this species are their exceptionally large mouths and powerful jaws. It would not be inappropriate to describe this frog as an enormous mouth with a body attached to it. The specific name is used as a noun in apposition.
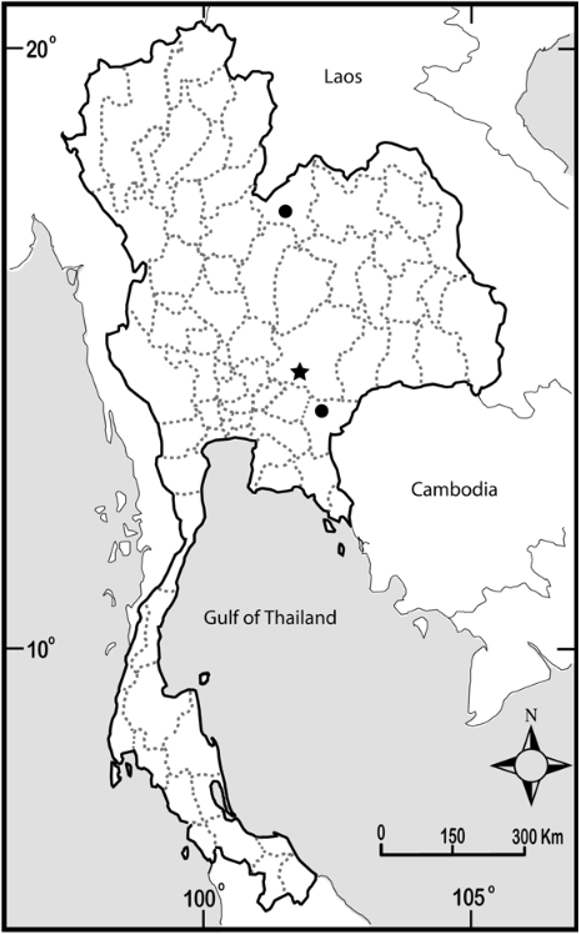
Distribution and ecology. Limnonectes megastomias is known from only three localities in eastern Thailand ( Fig. 6 View FIGURE 6 ). This aquatic frog inhabits streams in dry evergreen forest with year-round water supply and is rarely found away from water. Pang Si Da National Park, located on the southwestern margin of the Khorat Basin, was sampled at elevations between 90 and 600 m along roads, around buildings, in disturbed bamboo and evergreen forests, and in hill evergreen forest. Phu Luang Wildlife Sanctuary, located on the northwestern margin of the Khorat Basin, was sampled at elevation between 850 and 1460 m in hill evergreen, bamboo mixed with evergreen, and rhododendron heath forests. The last forest type was encountered at 1460 m on the summit of Kok Nok Kraba, where weather conditions were cool and foggy.
At SERS, located on the southwest margin of the Khorat Basin, this frog is known from only one closedcanopy stream. For most of the year, this spring-fed stream is reduced to standing pools connected by a continuous trickle of water. At the time of our visit, the pools were 10–15cm deep, except the largest pool at the head of the stream, was slightly less than 1m at the deepest point. Stream substrate is sand-silt overlaid with an accumulation of leaf detritus, under which these frogs are quick to seek cover when disturbed. Males were observed calling from 1400–2200 hrs. During two visits to the SERS site ( 2003 and 2005), only one other anuran ( Limnonectes gyldenstolpei ) was found to co-occur with L. megastomias . Clutches of newly deposited eggs were found in = 10 mm water at the SERS site on 15 March 2005. There was no evidence of parental egg care. Individual jelly capsules of eggs were 12–15 mm in diameter, with brown-pigmented ova 2.3–2.8 mm in diameter. Embryos maintained in captivity began hatching within 2 days of collection and had completed hatching after 7 days. Larvae had reached Gosner (1960) Stage 39 by 12 July when all remaining specimens were preserved. Limnonectes megastomias seems to be a sit-and-wait predator and is known to consume insects, frogs ( L. gyldenstolpei ), and birds.
The genetic distinctiveness of Limnonectes megastomias and the morphological differences that separate it from its congeners are certainly sufficient to warrant the recognition of this frog as a new species. There is, however, a frustrating amount of external morphological similarity between the new species and L. kuhlii , enough to warrant a brief discussion of the “ kuhlii complex” and some of the characters shared between L. kuhlii and L. megastomias .
Historically, the identification of Limnonectes kuhlii was credited to Dumeril and Bibron (1841) who provided a description of a single specimen from Java. Tschudi (1838), however, had previously (albeit briefly) described the same taxon under the same name, also from Java. Several names have been synonomized with L. kuhlii , including Rana conspicillata Günther (1872) from Sarawak (Borneo), Nyctibatrachus sinensis Peters (1882) from Guangzhou Province, China, Rana khasiana Boulenger (1882) from the Khasi Hills, India, and Rana paradoxa Mocquard (1890) from Sabah (Borneo) ( Frost, 2007).
Boulenger (1920) indicated that though he observed great intraspecific variation in Limnonectes kuhlii he was unable to find characters that defined “geographic races. ” Inger (1966) also noted variation among the populations of L. kuhii from Borneo, but found insufficient evidence using external morphology to warrant the recognition of separate species. While examining specimens of the “ kuhlii ” complex for this study, I have observed a great deal of similarity between L. megastomias and L. kuhlii , enough so that it would be difficult to identify individuals of the same approximate size to the level of species in the field.
Population-level variation in morphological characters (in particular, the presence and location of nuptial pads, and the degree to which the tympanum is obscured) is potentially confounding when one is trying to diagnose Limnonectes kuhlii and separate it from Limnonectes megastomias . All males of L. megastomias examined in this study (26 individuals from three populations; SVL 40–123 mm) all have nuptial pads on Fingers I and II. Inger (1966) noted that specimens of L. kuhlii from China and Thailand also possessed nuptial pads on Fingers I and II, whereas specimens from Sabah and Sarawak presented nuptial pads only on Finger I. These observations are corroborated by specimens I examined, with the exception of a single male from Thailand (Chiang Mai Province), which had nuptial pads only on Finger I. Additionally, specimens examined from Laos also have nuptial pads on Fingers II and III. Three adult males from Java examined in this study (sex determined by enlarged head and odontoids, and gonadal evidence): a poorly preserved and bleached lectotype (MNHN 4469), a paralectotype (RMNH 4297), and a recently preserved specimen. It is clear that the RMNH paralectotype and recent specimens lacks nuptial pads. It appears that the MNHN lectotype also lacks nuptial pads, but the poor state of preservation makes it difficult to say this with certainty. Examination of additional specimens hopefully will resolve this uncertainty.
Emerson & Berrigan (1993) described L. kuhlii , L. laticeps , and L. namiyei as all having concealed, but present, tympana. In general, the same character also diagnoses Limnonectes megastomias , yet in 12 of 37 specimens examined, the tympanic annulus was partially obscured, but visible enough to permit a measurement of the tympanic diameter. Similarly, Boulenger (1920), considered the tympanum of L. kuhlii hidden or slightly distinct, but provided measurements of the tympanic diameter. Inger (1966) noted that in Bornean populations of L. kuhlii , the tympanic annulus usually was not visible under the skin. Although Tschudi (1838) does not mention the tympanum of L. kuhlii, Duméril and Bibron (1841) discuss its size in relation to the upper eyelid, presumably visible and measurable without dissection of the single Javan specimen from which they based their description. In Javan specimens of L. kuhlii examined here, 12 of 13 have obscured tympana, but still visible enough to allow for measurement. The tympanic annulus is not visible in the poorly preserved lectotype. The specimens of L. kuhlii that I examined from Thailand and Laos also varied in the degree to which the tympanum was obscured (obscure, but visible in 7 of 14, and 4 of 5 specimens, respectively), sometimes even varying from side to side in the same animal. It is possible that the state of preservation and hydration of a specimen affects the degree to which the tympanic annulus is visible through the skin. Because most systematic works are based on fluid-preserved specimens, this may influence how this character appears to the investigator. It is reasonable to consider that as a specimen dries, the skin covering of the head covering the tympanic structures tightens and presents an “obscure, but visible” tympanic annulus. The variation in this character among populations of L. kuhlii and its allies warrants closer examination (both in preserved and live specimens).
The presence of bifid subarticular tubercles (proximal or distal, or both) on Fingers II or III (or both) was noted only in some specimens of Limnonectes megastomias . This same character was also observed in four individuals of L. kuhlii from Chiang Mai ( Thailand), one specimen of L. kuhlii from Laos, and in none of the specimens from Java. In the Chiang Mai population, only Finger III has bifid tubercles (proximal in all cases, distal in one). The specimen from Laos has a bifid distal subarticular tubercle on Finger III. It is interesting that in the SERS population of L. megastomias bifid subarticular tubercles are present in all individuals examined, but in other populations it seems to be highly variable. The apparent small size and isolation of the SERS population to only one stream may affect the frequency of inheritance of this trait.
In Thailand, Limnonectes kuhlii is distributed along the mountainous western edge of the Kingdom from north to south, including the central portion of the Thai-Malay Peninsula ( Chan-ard, 2003). The type locality of L. megastomias and Pang Si Da National Park are more than 400 km distant from the western edge of the known distribution of L. kuhlii in Thailand. There are records, however, of L. kuhlii (a small-bodied form; SVL < 90 mm) from Loei Province where specimens of L. megastomias also were collected (Phu Luang Wildlife Sanctuary). The amount of genetic divergence between samples of L. kuhlii from Chiang Mai Province ( Thailand) and L. kuhlii from Java ( type locality) (14–16%) would suggest that what is currently recognized, as “ kuhlii ” in Thailand is a distinct species. Furthermore, based on the genetic distance between L. megastomias and Chiang Mai populations of L. kuhlii (±9%), it is plausible that there are multiple species of “ kuhlii ”-like frogs in Thailand and that some of those populations may occur sympatrically with L. megastomias . Because of the wide distribution of L. kuhlii and the broad range of morphological variation, it is also possible that populations currently recognized, as L. kuhlii will be subsumed by L. megastomias . A broad and robust sampling of specimens from across the known distribution of L. kuhlii is currently underway to investigate the molecular structure of this species at the population level (McLeod, in prep).
There remains a great deal of work necessary to resolve fully the identity of the frogs in the kuhlii -complex. Phylogenetic resolution of this enigmatic group, and other groups like it, will not be possible without the detailed study of internal and external morphology coupled with a robust molecular analysis (McLeod, in prep).
TABLE 3. Measurements of tadpole series (FMNH 266340). See text for character definitions. All larvae were staged based on Gosner (1960). * indicates the stage 40 tadpole illustrated in Figure 5.
| Stage 28 | 30 | 30 | 31 | 32 | 34 | 34 | 36 | 40* | 40 | 40 | 40 | 41 | 41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL 10.2 | 10.3 | 11.1 | 11.0 | 11.2 | 12.1 | 11.0 | 13.8 | 14.4 | 13.7 | 13.1 | 13.6 | 13.7 | 12.9 |
| TL 17.8 | 20.8 | 20.4 | 20.7 | 22.0 | 20.0 | 18.8 | 23.2 | 28.0 | 23.5 | 26.8 | 25.9 | 30.3 | 30.8 |
| ToL 28.0 | 31.1 | 31.5 | 31.8 | 33.3 | 32.1 | 29.8 | 37.0 | 42.4 | 37.2 | 40.0 | 39.5 | 44.0 | 43.7 |
| BW 4.7 | 5.2 | 5.3 | 5.3 | 5.4 | 5.9 | 5.2 | 7.6 | 8.5 | 8.1 | 7.4 | 7.4 | 7.5 | 7.0 |
| BH 3.5 | 2.6 | 2.6 | 3.5 | 3.5 | 2.6 | 3.6 | 3.6 | 3.5 | 3.8 | 2.8 | 4.0 | 3.8 | 4.0 |
| LED 1.3 | 1.3 | 1.4 | 1.5 | 1.5 | 1.5 | 1.5 | 1.8 | 1.8 | 1.8 | 1.5 | 1.8 | 1.8 | 1.7 |
| LIO 2.5 | 2.3 | 2.7 | 2.7 | 2.7 | 2.8 | 2.6 | 3.0 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.6 |
| LIN 1.8 | 1.8 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 2.3 | 2.2 | 2.1 | 2.0 | 2.0 | 1.8 | 1.7 |
| LNS 1.5 | 1.0 | 1.8 | 1.5 | 1.7 | 1.8 | 1.3 | 1.8 | 1.8 | 2.0 | 1.4 | 2.0 | 1.5 | 1.5 |
| LEN 1.1 | 1.1 | 1.2 | 1.1 | 1.2 | 1.3 | 1.1 | 1.3 | 1.4 | 1.5 | 1.5 | 1.2 | 1.3 | 1.3 |
| SW 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.5 | 0.7 | 0.8 | 0.8 | 0.8 | 0.5 | 1.0 | 0.7 | 1.0 |
| TMH1 2.8 | 2.8 | 2.8 | 2.8 | 3.0 | 3.0 | 3.0 | 3.5 | 3.5 | 3.5 | 3.3 | 3.5 | 3.3 | 3.3 |
| TMH2 2.0 | 2.5 | 2.5 | 2.5 | 2.4 | 2.5 | 2.7 | 2.9 | 3.3 | 3.0 | 2.8 | 3.4 | 2.0 | 2.5 |
| TH 4.9 | 8.0 | 5.3 | 5.2 | 5.5 | 5.8 | 5.5 | 7.3 | 7.0 | 8.0 | 6.0 | 6.8 | 8.0 | 5.6 |
| BH/BL 0.34 | 0.25 | 0.24 | 0.31 | 0.31 | 0.21 | 0.32 | 0.26 | 0.24 | 0.27 | 0.21 | 0.30 | 0.28 | 0.31 |
| BL/ToL 0.36 | 0.33 | 0.35 | 0.35 | 0.34 | 0.38 | 0.37 | 0.37 | 0.34 | 0.37 | 0.33 | 0.34 | 0.31 | 0.29 |
| BW/BL 0.46 | 0.51 | 0.48 | 0.48 | 0.48 | 0.49 | 0.47 | 0.55 | 0.59 | 0.59 | 0.56 | 0.55 | 0.55 | 0.54 |
| LED/BL 0.12 | 0.12 | 0.13 | 0.13 | 0.13 | 0.12 | 0.14 | 0.13 | 0.12 | 0.13 | 0.11 | 0.13 | 0.13 | 0.13 |
| LED/BH 0.36 | 0.48 | 0.53 | 0.42 | 0.41 | 0.56 | 0.42 | 0.49 | 0.50 | 0.47 | 0.53 | 0.44 | 0.46 | 0.41 |
| LIO/BW 0.53 | 0.43 | 0.50 | 0.50 | 0.49 | 0.47 | 0.50 | 0.39 | 0.41 | 0.43 | 0.47 | 0.47 | 0.46 | 0.51 |
| LIN/LIO 0.70 | 0.78 | 0.72 | 0.70 | 0.72 | 0.69 | 0.73 | 0.76 | 0.61 | 0.60 | 0.57 | 0.57 | 0.51 | 0.46 |
| TL/ToL 0.64 | 0.67 | 0.65 | 0.65 | 0.66 | 0.62 | 0.63 | 0.63 | 0.66 | 0.63 | 0.67 | 0.66 | 0.69 | 0.71 |
| TL/BL 1.75 | 2.02 | 1.83 | 1.88 | 1.96 | 1.65 | 1.71 | 1.67 | 1.95 | 1.72 | 2.04 | 1.90 | 2.22 | 2.39 |
| TH/ToL 0.17 | 0.26 | 0.17 | 0.16 | 0.17 | 0.18 | 0.18 | 0.20 | 0.17 | 0.22 | 0.15 | 0.17 | 0.18 | 0.13 |
| TH2/TH 0.41 | 0.31 | 0.48 | 0.48 | 0.44 | 0.43 | 0.49 | 0.40 | 0.46 | 0.38 | 0.46 | 0.50 | 0.25 | 0.45 |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |