Leptalpheus marginalis Anker, 2011
|
publication ID |
https://doi.org/10.11646/zootaxa.4766.1.3 |
|
publication LSID |
urn:lsid:zoobank.org:pub:7670778E-F123-4ACF-A349-A04B426FD876 |
|
DOI |
https://doi.org/10.5281/zenodo.3803797 |
|
persistent identifier |
https://treatment.plazi.org/id/124D87CD-FFB8-FFFF-C6EA-E5C6D0BCB437 |
|
treatment provided by |
Carolina |
|
scientific name |
Leptalpheus marginalis Anker, 2011 |
| status |
|
Leptalpheus marginalis Anker, 2011 View in CoL
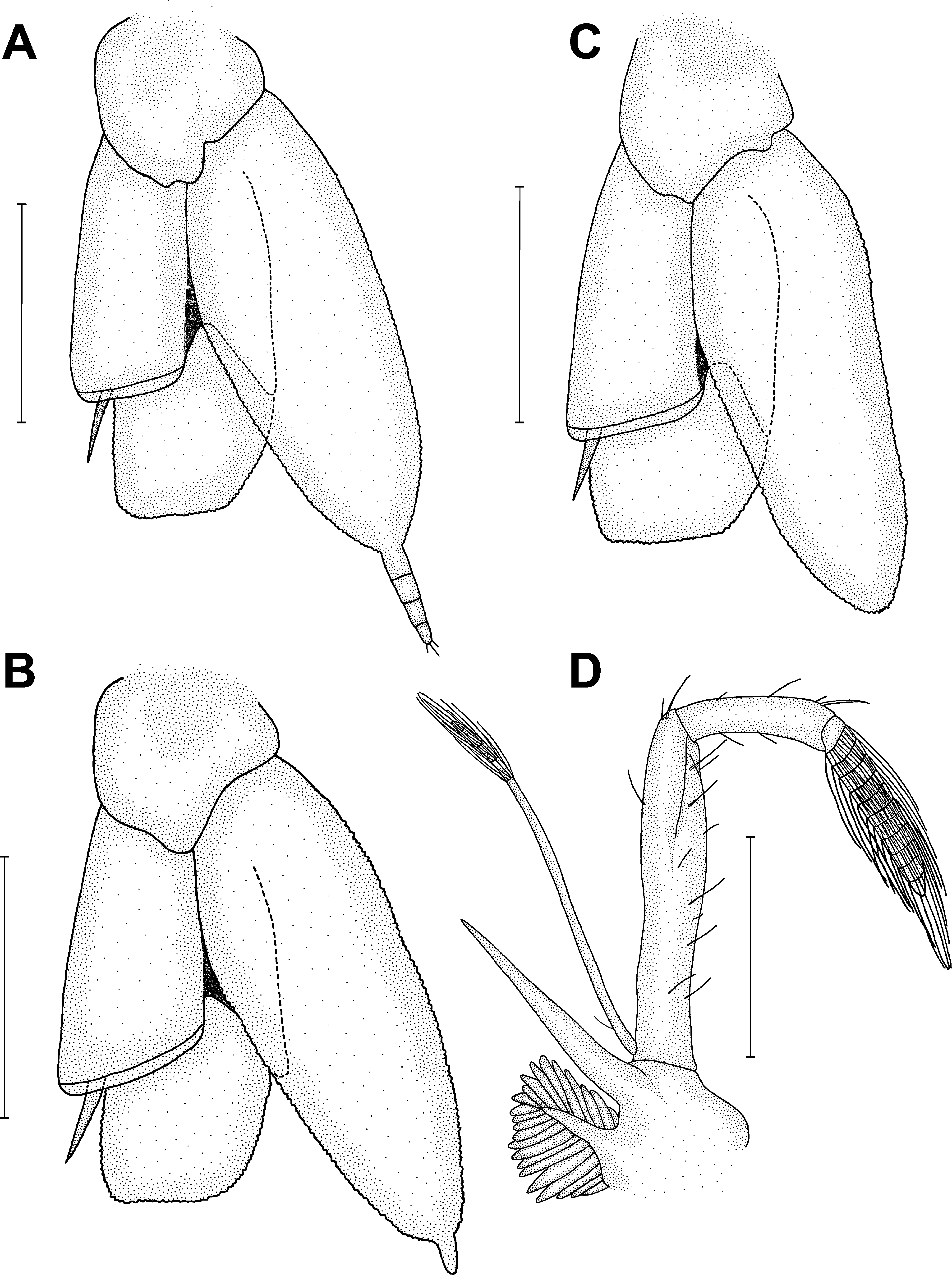
Figures 8–11 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11
Leptalpheus marginalis View in CoL — Anker 2011: 4–6 View Cited Treatment , figs. 1, 2.
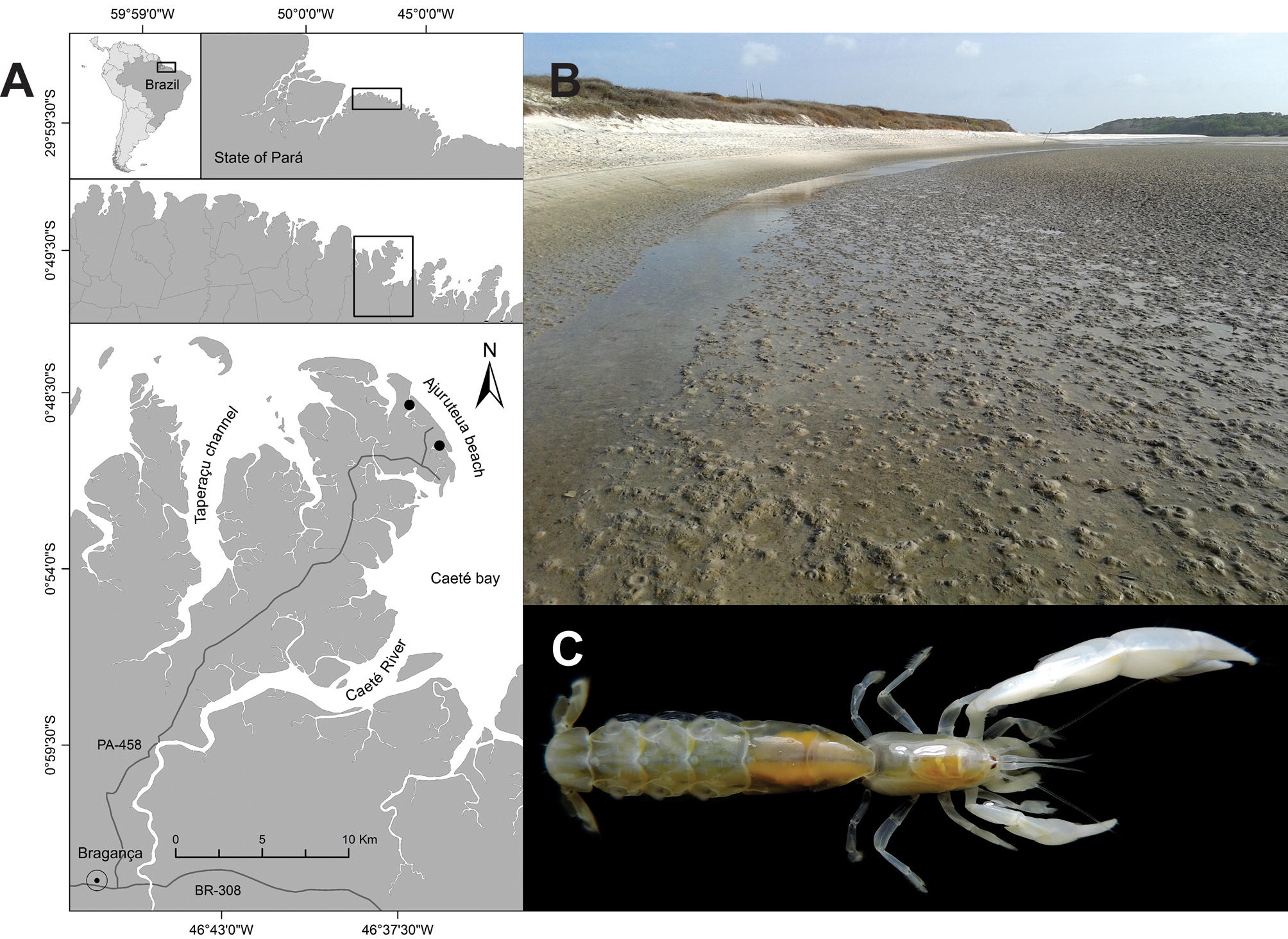
Material examined. 10 males (CL=4.33 ± 0.61 mm; TL=11.19 ± 1.63 mm), two non-ovigerous females (CL=4.52 ± 0.68 mm; TL=11.98 ± 0.2 mm), and three ovigerous females (CL=4.49 ± 0.2 mm; TL=11.69 ± 0.2 mm); muddysandy intertidal zone of the Ajuruteua Peninsula ( Fig. 1A, B View FIGURE 1 ) in the Bragança region of the state of Pará, northern Brazil ( 046°37’8.13”W, 0°48’52.92”S; 046°36’11.81”W, 0°50’9.10”S; 046°36’9.80”W, 0°50’11.22”S); 23 June 2013, 3 March 2014, 11 April 2015, 19 March 2017, 20 May 2017, and 13 April 2018.
Description. See Anker (2011).
Density and population structure. L. marginalis was less abundant than L. forceps , and was encountered only rarely in the study area. In fact, only 48 specimens were captured from the 3000 L. siriboia burrows sampled during the present study. From the total number of L. marginalis specimens collected, 38 individuals were identified as males (79.2%) and 10 as females (20.8%), resulting in an overall sex ratio of 3.8M: 1F. Eight (80% of the total) of the females had eggs in their abdomens. The density of L. marginalis ranged from 0 to 1 specimen per callichirid burrow.
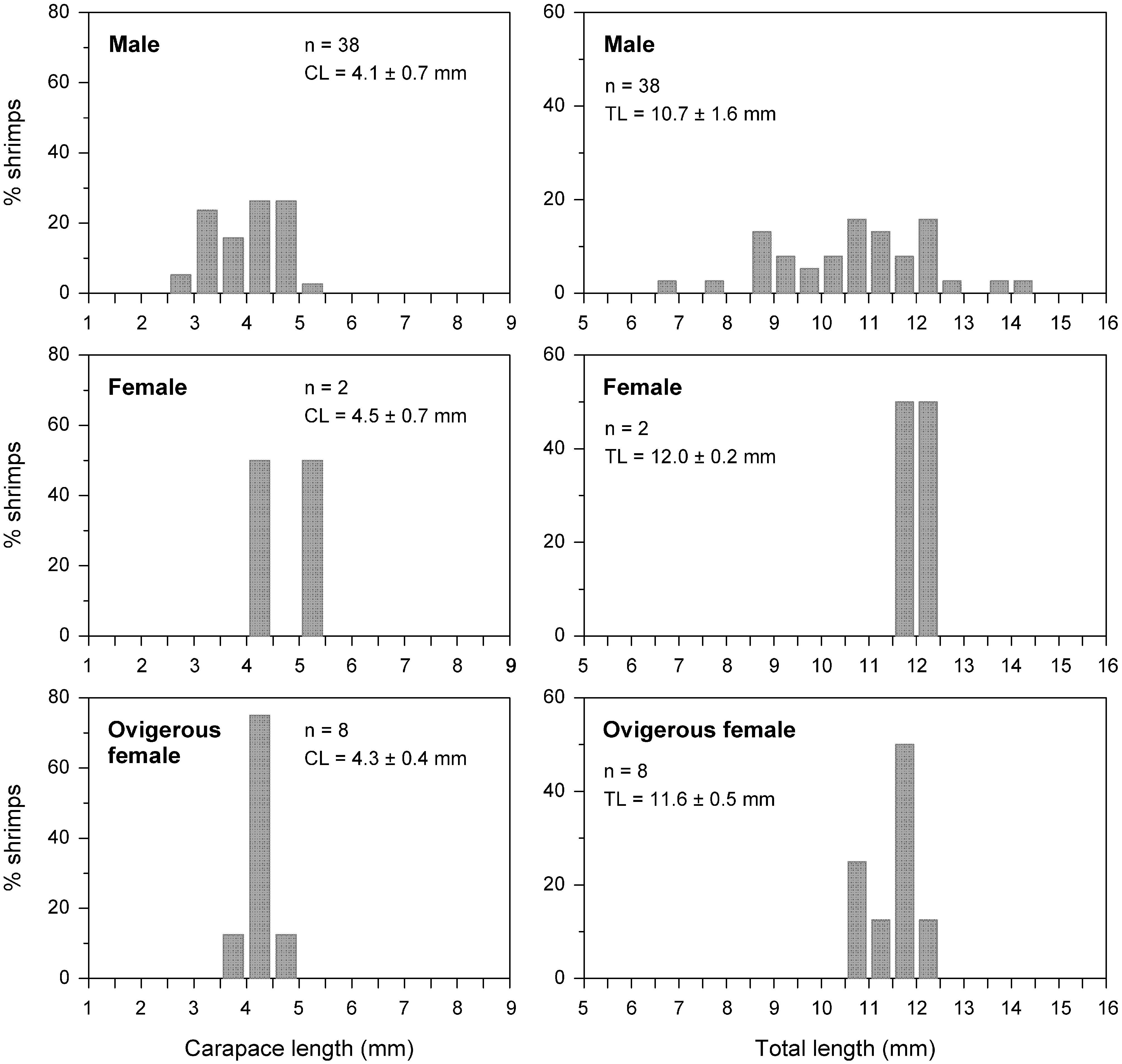
The carapace lengths (CL) of the male shrimps ranged from 2.6 to 5.2 mm (4.1 ± 0.7 mm), with the highest percentages of specimens being recorded in the 3.0– 3.5 mm (23.7%) size class and in the classes between 4.0 and 5.0 mm CL (26.3%) ( Fig. 12 View FIGURE 12 ). The non-ovigerous females (n=2) had CLs of 4.0 and 5.0 mm, while the CLs of the ovigerous specimens ranged from 3.5 to 4.7 mm (4.3 ± 0.4 mm), with most (75%) of the individuals being recorded in the 4.0– 4.5 mm size class ( Fig. 12 View FIGURE 12 ).
The male shrimps presented total body lengths (TL) ranging from 6.7 to 14.1 mm (10.7 ± 1.6 mm), with the highest frequencies of the specimens being grouped in the 8.5–9.0 mm (13.2%) size class, in the classes between 10.5 and 11.5 mm TL (13.2 and 15.8%), and in the 12.0– 12.5 mm (15.8%) size class ( Fig. 12 View FIGURE 12 ). The two non-ovigerous females had TLs of 11.8 and 12.1 mm. The TLs of the ovigerous specimens ranged from 10.6 to 12.1 mm (11.6 ± 0.5 mm) and most of the specimens were included in the 10.5–11.0 mm (25%) and 11.5–12.0 mm (50%) size classes ( Fig. 12 View FIGURE 12 ).
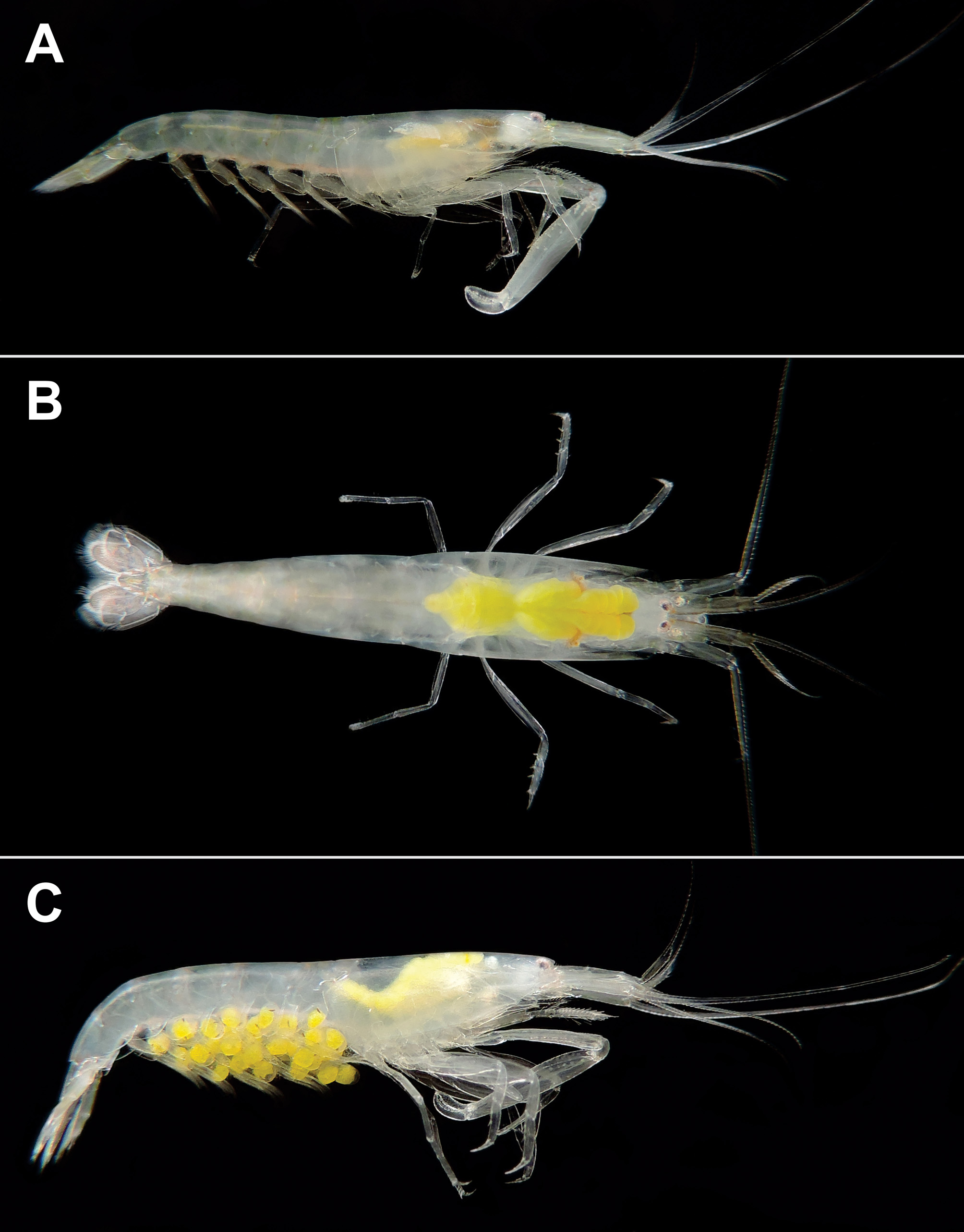
Colour in life. Body semitransparent, with numerous reddish or purplish chromatophores forming diffuse bands around the abdomen; hepatopancreas greenish or orange in colour; the posterior margin of the abdominal somites with greenish chromatophores; antennae, antennules, tail fan, and chelipeds with concentrations of greenish or reddish chromatophores ( Fig. 8 View FIGURE 8 A–C); eggs bright green in early stages of development and semitransparent when close to hatching ( Fig. 8C View FIGURE 8 ).
......continued on the next page
......continued on the next page
......continued on the next page
Distribution. Previously known only from the type locality on the Caribbean coast of Colombia (see Anker 2011). Now also known from the Ajuruteua Peninsula in the Bragança region, northeastern coast of the state of Pará, Amazon region, Brazil (present study).
Ecology. The present study represents the first report of the presence of L. marginalis living in burrows of the callichirid “ghost” shrimp, L. siriboia , in a muddy-sandy intertidal habitat in northern Brazil ( Fig. 1 View FIGURE 1 A–C). Given the cryptic life style of this and other Leptalpheus species, data on their feeding behaviour, ecology, breeding season, growth, and embryonic and larval development are still non-existent, and require further investigations.
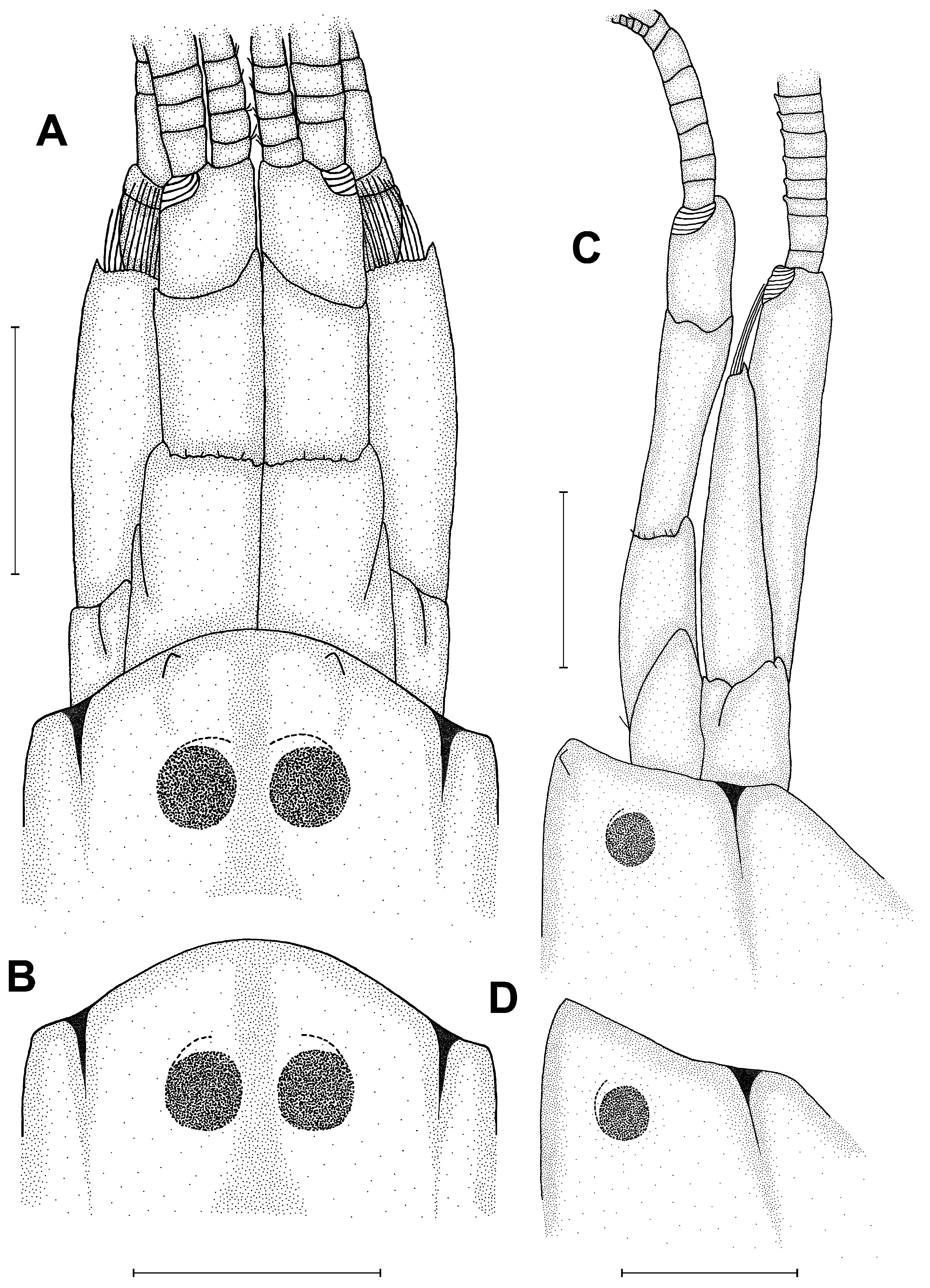
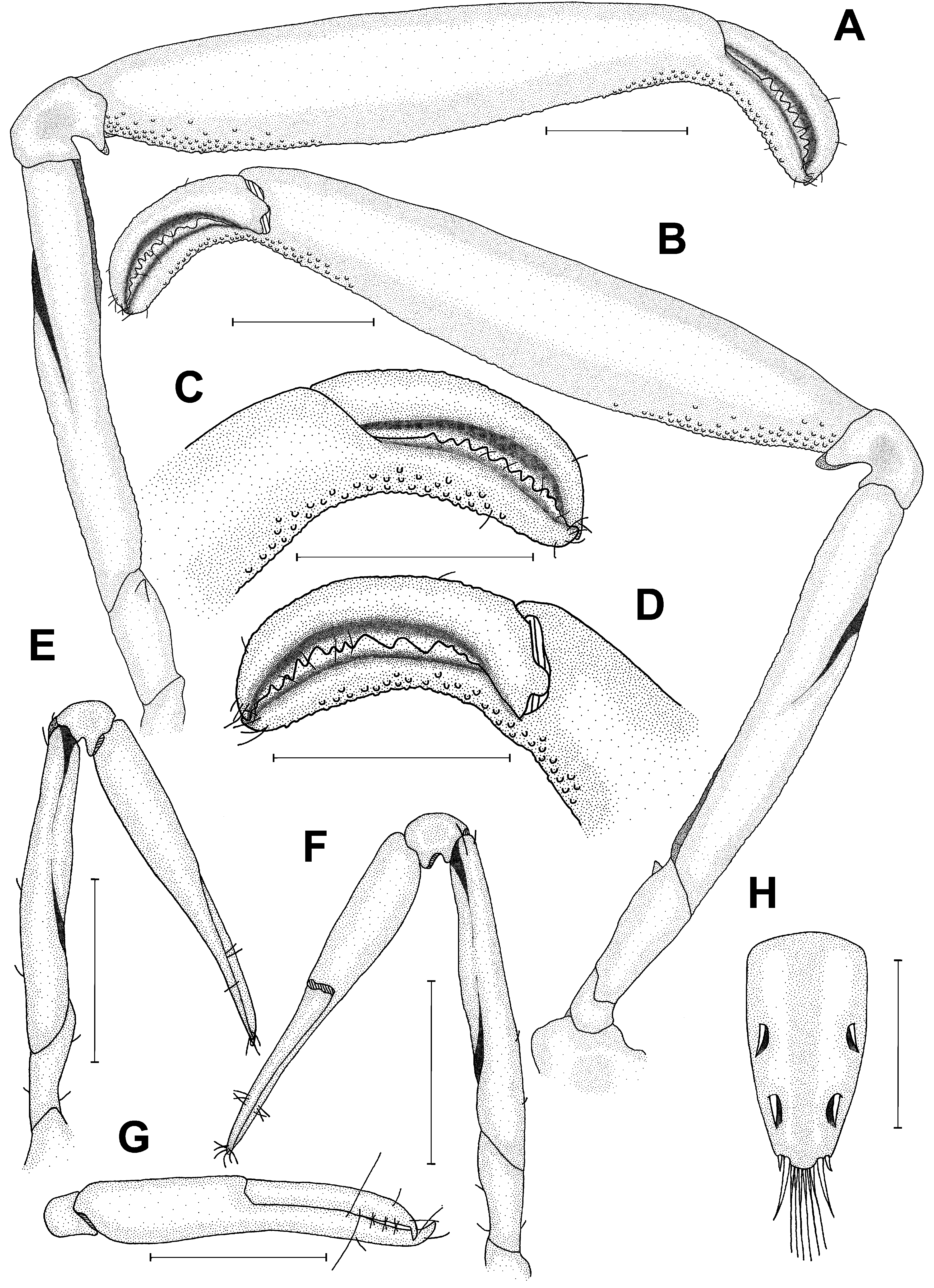
Remarks. In the present study, L. marginalis of both sexes from the Ajuruteua Peninsula, Amazon region, were analyzed and compared with Anker’s (2011) description of the male holotype from the coast of Colombia. According to Anker (2011), L. marginalis can be differentiated from other Leptalpheus species primarily by the lack of a gap when the fingers of the major chela are closed ( Fig. 10C, D View FIGURE 10 ; Anker 2011, fig. 2E). The morphology of the Amazonian specimens is broadly consistent with that of the male holotype from Colombia. However, L. marginalis from the Amazon region have more robust antennular articles and a slightly more rounded frontal margin of the carapace (see Fig. 9A, B View FIGURE 9 ) than the Colombian holotype (see Anker 2011, fig. 1A). Also, our specimens showed a third maxilliped slightly more elongated with an exopod 5-segmented and an ultimate segment divided in 12 narrow segments (see Fig. 11D View FIGURE 11 ) in contrast to L. marginalis described by Anker (2011, fig. 1I). Other important differences are related to the presence or absence of orbital crests in front of the eyes ( Fig. 9A, B View FIGURE 9 ; Anker 2011, fig. 1A–C), and of an appendix (i.e., caudal filament) on each uropodal endopod, sometimes segmented or not ( Fig.11 View FIGURE 11 A–C; Anker 2011, fig. 1M). In the Amazonian specimens, the presence or absence of these orbital crests or caudal filaments was randomly recorded in both the larger adults and juveniles of both sexes. The presence of orbital crests situated near the anterolateral margin of the carapace was also reported in the literature for other Leptalpheus specie s such as Leptalpheus bicristatus from Chame Bay, Pacific coast of Panama ( Anker 2011, fig. 15A, B), L. felderi from Isla Margarita, Venezuela, and Bahía Cispata, Colombia ( Anker et al. 2006b, fig. 1A–C), and L. mexicanus from Estero de Urías, Mazatlán, Sinaloa, Mexico ( Salgado-Barragán et al. 2014, fig. 6A, B, E, F). Caudal filaments on uropodal endopods have also been described in L. cf. forceps from Costa Rica ( Anker 2008, fig. 4J), in L. forceps from Ajuruteua Peninsula, Brazil (present study, Fig. 3D View FIGURE 3 ), and in L. felderi from Venezuela ( Anker et al. 2006b, fig. 5C).
It is likely that the L. marginalis from the coast of Colombia could also present the same pattern of variation found in the Amazonian specimens. The fact that Anker (2011) has analyzed only three male specimens, the morphological characters described by this author may not be conclusive, and more specimens should be analyzed to elucidate these differences. The marginal crests were described by Anker (2011) as one of a number of taxonomic characters used for differentiating L. marginalis from other Leptalpheus species (e.g., L. forceps , L. mexicanus , Leptalpheus pierrenoeli , and Leptalpheus axianassae ). The variation in the presence of orbital crests observed in our study may therefore indicate an intraspecific variability among geographically isolated populations and these structures should not longer be considered as a good morphological character for phylogenetic separation. This variation was also documented by Salgado-Barragán et al. (2014) for L. mexicanus . These authors suggested the exclusion of these orbital crests as distinctive character between L. mexicanus and L. bicristatus .
The functional morphology of the orbital crests and the caudal filaments in L. marginalis and other congeneric species is still unclear. The presence or absence of these structures may reflect either phenotypic adaptations to the environment or genetic variation. Given the considerable morphological variation found in the genus Leptalpheus , further research is required to determine whether this intraspecific variation has a genetic basis.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Caridea |
|
Family |
|
|
Genus |
Leptalpheus marginalis Anker, 2011
| Rosário, Tayse N., Pires, Marcus A. B., Souza, Adelson S., Fernandes, Marcus E. B., Abrunhosa, Fernando A. & Simith, Darlan J. B. 2020 |