Tenuibiotus zandrae, Stec & Tumanov & Kristensen, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.614 |
|
publication LSID |
lsid:zoobank.org:pub:8F2CC4F8-D4F8-4870-A0C7-3D8D78F2D5EC |
|
DOI |
https://doi.org/10.5281/zenodo.3718297 |
|
persistent identifier |
https://treatment.plazi.org/id/99D6163D-E8E6-4223-B975-F68AA2268304 |
|
taxon LSID |
lsid:zoobank.org:act:99D6163D-E8E6-4223-B975-F68AA2268304 |
|
treatment provided by |
Plazi |
|
scientific name |
Tenuibiotus zandrae |
| status |
sp. nov. |
Tenuibiotus zandrae View in CoL sp. nov.
urn:lsid:zoobank.org:act:99D6163D-E8E6-4223-B975-F68AA2268304
Figs 10–19 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Etymology
We take great pleasure in dedicating this new species to the friend of the first and third authors. Zandra Maria Skandrup Sigvardt, who recently completed her PhD studies working on crustaceans (Section of Biosystematics) at the Natural History Museum of Denmark in Copenhagen.
Material examined
62 animals (including 18 simplex) and 171 eggs. Specimens mounted on microscope slides in Hoyer’s medium (49 animals + 161 eggs), fixed on SEM stubs (including two extracted buccal apparatuses) (10 +10), and processed for DNA sequencing (3 +0).
Holotype
GREENLAND • Disko Island , Østerlien; 69°15′17″ N, 53°30′46″ W; 30 m a.s.l.; sample of moss collected from the rock in arctic tundra; IZiBB, slide GL.011.17 . GoogleMaps
Paratypes
GREENLAND • 58 paratypes; same collection data as for holotype; IZiBB, slides GL.011.08 to 011.09, 011.16 to 011.19, 011.22 to 011.23, 011.27 to 011.28, SEM stubs GL.012.06 to 012.07, 012.13 GoogleMaps • 171 eggs; same collection data as for holotype; IZiBB, slides GL.011.10 to 011.15, 011.20 to 011.21, 011.24 to 011.26, 011.29, SEM stub GL.12.13 GoogleMaps .
Description
Animals (measurements and statistics in Table 4)
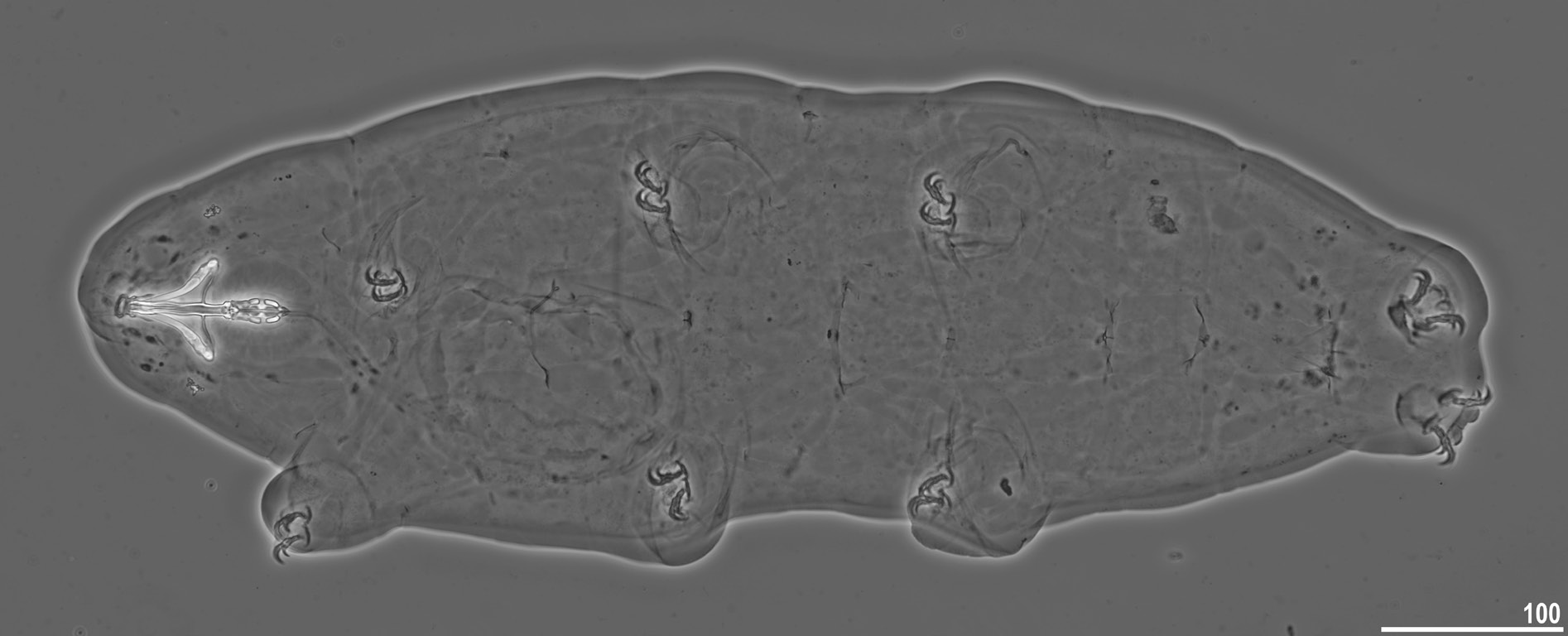
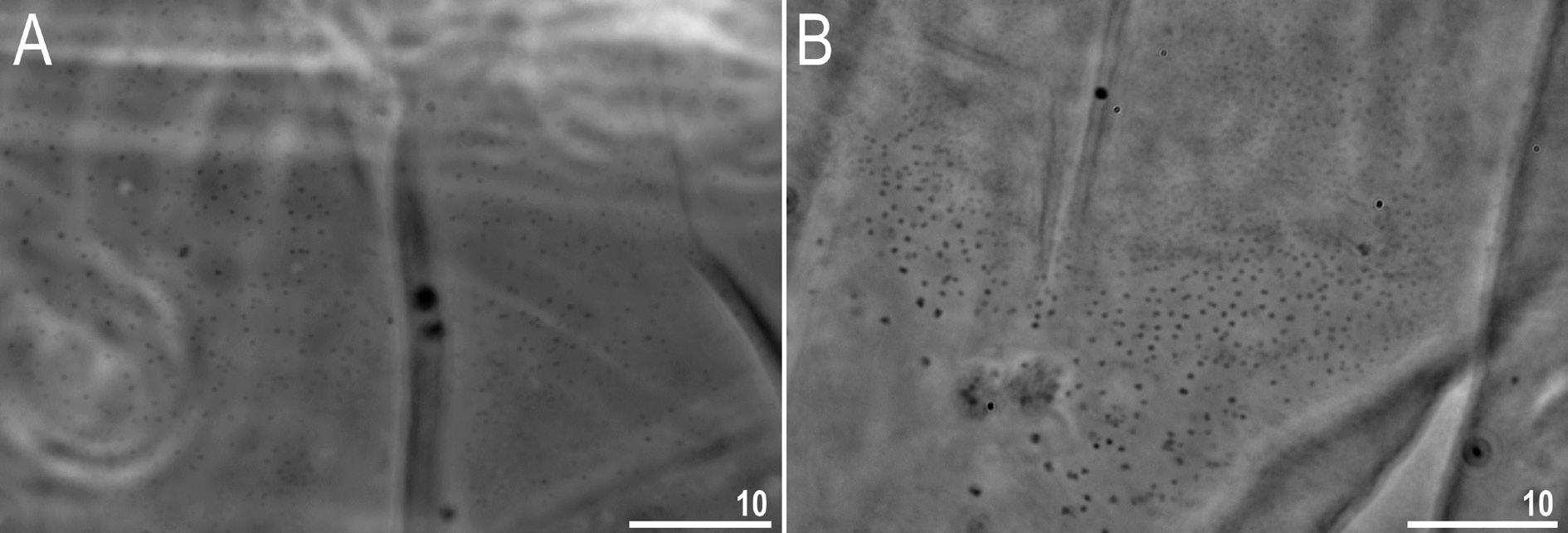
Body transparent in juveniles and whitish in adults, after fixation in Hoyer’s medium transparent ( Fig. 10 View Fig ). Eyes present in specimens mounted in Hoyer’s medium. Body cuticle without pores but covered with fine granulation including ventral side of the body and all legs ( Figs 11–13 View Fig View Fig View Fig ). Granulation is distributed uniformly on the body ( Fig. 11 View Fig A–B, E) but sometimes, especially in larger specimens, random patches without granulation are present on the body cuticle ( Fig. 11 View Fig C–D, F). Patches of dense granulation composed of cushions with aggregated granules present on all legs ( Figs 12–13 View Fig View Fig ). A patch of clearly visible granulation, is present on the external surface of legs I–III just below the claws ( Figs 12A View Fig , 13A View Fig ). A pulvinus is absent on the internal surface of legs I–III, whereas a patch of dense granulation is present and wider than the patch on the external leg surface ( Figs 12B View Fig , 13B View Fig ). A patch of dense granulation on legs IV is always visible and covers the dorsal and the lateral sides of hind legs ( Figs 12C View Fig , 13 View Fig C–D). Claws slender, of the Tenuibiotus type ( Fig. 14 View Fig ). Primary branches with distinct accessory points, a long common tract, and with an evident stalk connecting the claw to the very wide lunula ( Fig. 14 View Fig ). Lunulae I–III smooth ( Fig. 14A, C View Fig ), whereas lunulae IV exhibit clear dentation ( Fig. 14B, D View Fig ). The horseshoe structure connecting the anterior and the posterior claw is present and is visible only in PCM ( Fig. 14B View Fig ).
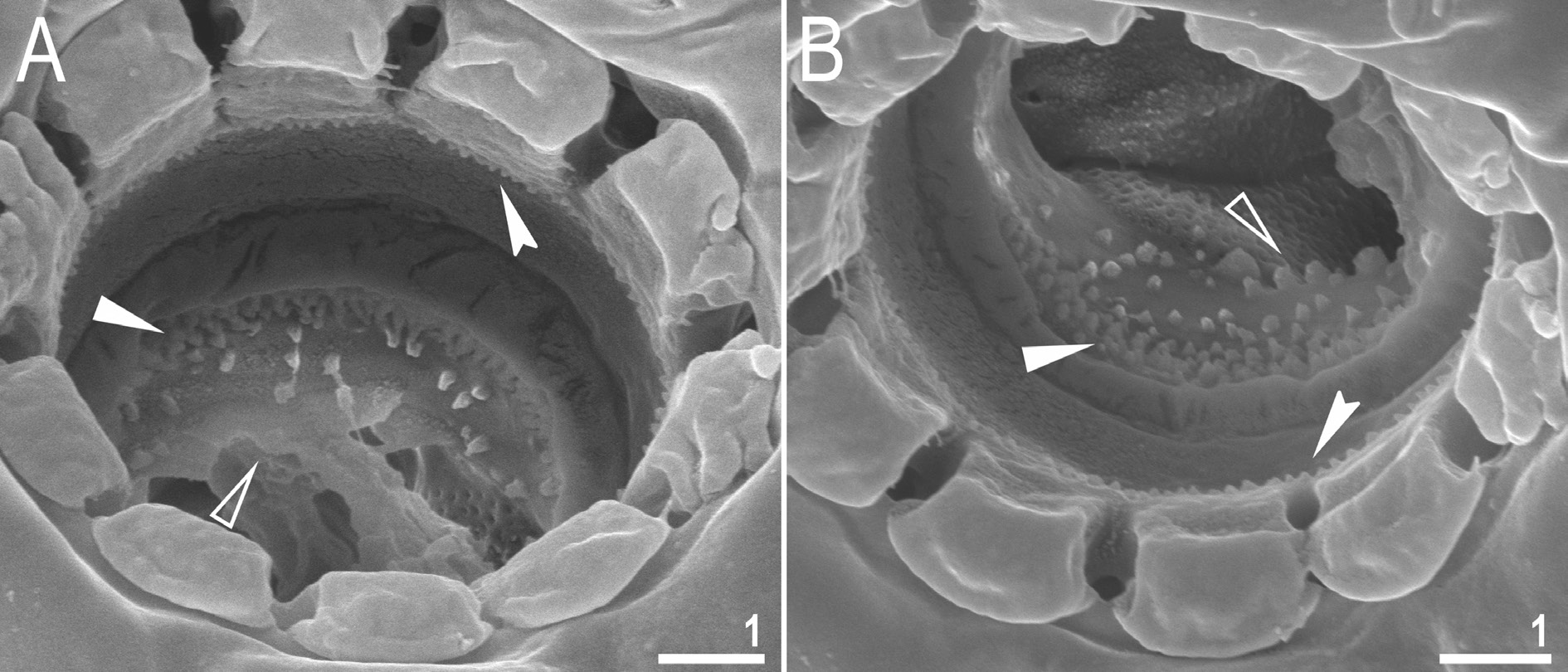
Mouth antero-ventral, followed by ten peribuccal lamellae ( Figs 15A View Fig , 16 View Fig ). Bucco-pharyngeal apparatus of the Macrobiotus type ( Figs 15A View Fig , 17A View Fig ). Under LCM, only the second and third bands of teeth visible, with the second band being faintly marked ( Fig. 15 View Fig B–C). However, in SEM all three bands of teeth are visible, with the first band being situated at the base of peribuccal lamellae and composed of 1–2 rows of small, cone-shaped teeth arranged around the oral cavity ( Figs 16 View Fig , 17B View Fig ). The second band of teeth is situated between the ring fold and the third band of teeth and comprises 3–6 rows of small, cone-shaped teeth ( Figs 15 View Fig B–C, 16). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the buccal tube opening ( Figs 15 View Fig B–C,16). The third band of teeth is discontinuous and divided into dorsal and ventral portions. Under LCM, the dorsal teeth are seen as three distinct transversal ridges of which the median tooth is triangular and is wedged between the lateral teeth ( Fig. 15B View Fig ). The ventral teeth under LCM appear as three to four separate roundish teeth, largest than those of the second band ( Fig. 15C View Fig ), only sometimes they can be seen as one faintly marked, elongated tooth. In SEM, both dorsal and ventral teeth are also clearly distinct ( Fig. 16 View Fig ). Under SEM, the medio-dorsal tooth is the largest within the third band and is positioned anteriorly with respect to the lateral teeth ( Fig. 16A View Fig ), whereas the ventral portion consist of cone-shaped teeth of which the lateral ones are larger than the medial ones ( Fig. 16B View Fig ). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and a small triangular microplacoid ( Fig. 15A View Fig , D–E). The macroplacoid length sequence is 2<1. The first macroplacoid exhibits central constriction whereas the second macroplacoid is sub-terminally constricted ( Figs 15 View Fig D–E, 17C).
Eggs (measurements and statistics in Table 5)
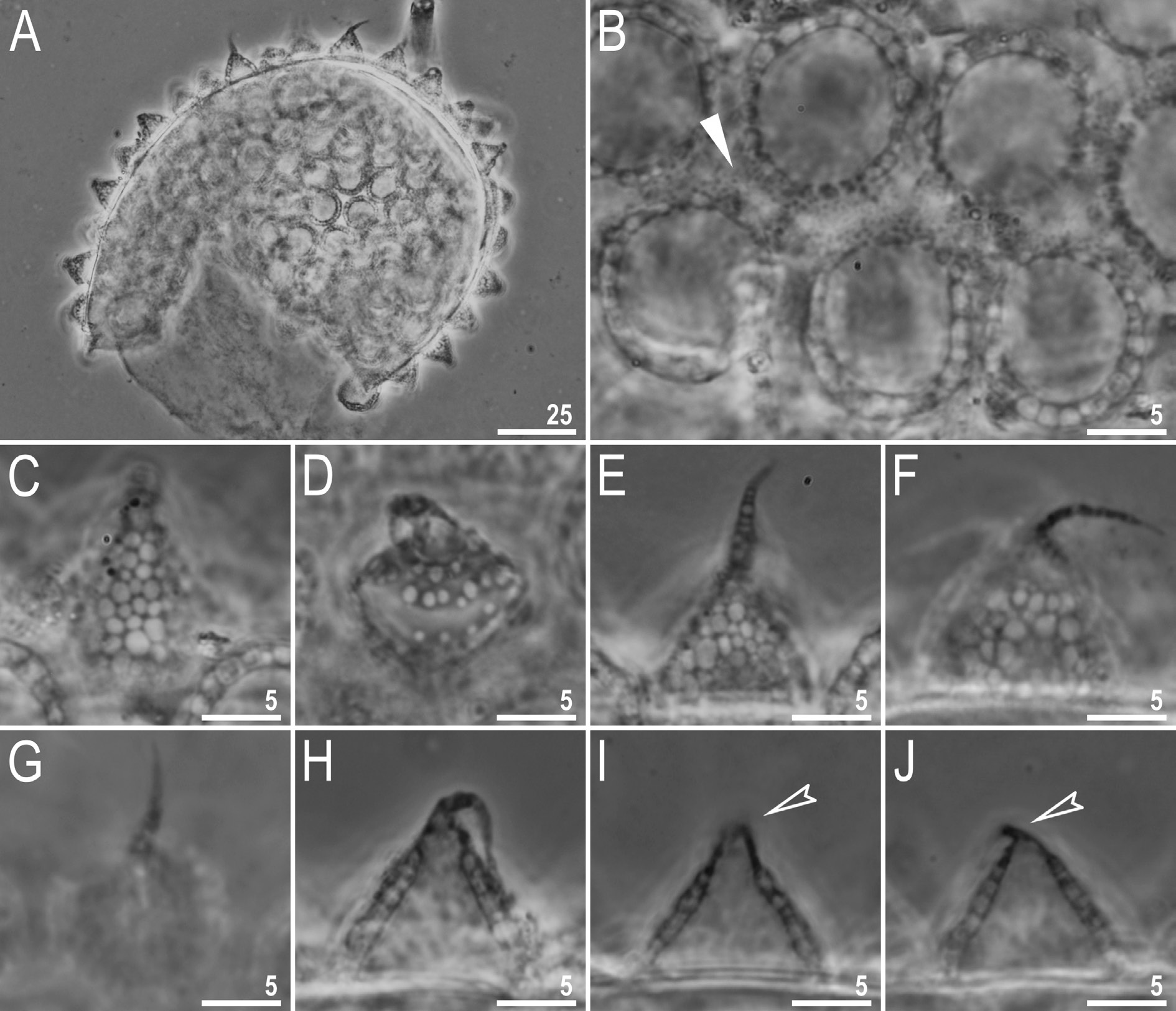
Laid freely, whitish, spherical or ovoid ( Figs 18 View Fig A–B, 19A). The surface between processes is smooth, with thickenings/striae often radiating from the processes bases ( Figs 18 View Fig B–D, 19B–C, E–F). Under PCM, these thickenings together with labyrinthine layer within chorion are visible as dark dots and lines on the surface between processes, whereas under SEM they are smooth striae coming out of the process bases ( Figs 18 View Fig B–D and 19B–C, E–F, respectively). Under SEM, the surface between processes and between the peribasal striae is covered with micropores ( Fig. 19 View Fig E–F). Processes are of conical shape, with elongated apices which are sometimes bi- or trifurcated ( Figs 18 View Fig E–H, 19A–D). The labyrinthine layer between the process walls is clearly visible under LCM as a reticular pattern with sinuous margins ( Fig. 18 View Fig C–D). The elongated meshes decrease in size from the base to the top of the processes ( Fig. 18 View Fig C–D). Under SEM, the surface of the processes is covered with small tubercles, whereas the surface of the elongated apices is smooth ( Fig. 18 View Fig B–E).
Reproduction
The examination of specimens freshly mounted in Hoyer’s medium did not revealed any spermathecae or testes filled with spermatozoa. Also, male secondary sexual dimorphism traits such as lateral gibbosities on legs IV were absent. Thus, reproductive mode could not be unambiguously determined.
DNA sequences
We obtained sequences of good quality for all four of the above-mentioned DNA markers. Sequences of each marker were represented by single haplotypes:
18S rRNA sequence (GenBank: MN443040 View Materials ), 1035 bp long;
28S rRNA sequence (GenBank: MN443035 View Materials ), 780 bp long;
ITS-2 sequence (GenBank: MN443038 View Materials ), 439 bp long;
COI sequence (GenBank: MN444827 View Materials ), 658 bp long.
Morphological observations of comparative material
Amended description of Tenuibiotus voronkovi
The examination of the holotype and paratype of T. voronkovi under LCM revealed the presence of granulation on the body cuticle. Faint granulation with small and uniformly sized granules is regularly arranged and covers the dorso-medial region of the body, from its cephalic to the caudal end ( Fig. 20A View Fig ). On the dorso-lateral surface of the body, granulation is unevenly distributed and resembles patches of granules, within which granule size gradually increases in the dorsal to lateral direction ( Fig. 20B View Fig ). The granulation is absent or not visible in LCM on the ventral side of the body. Similarly, the granulation is absent on the legs except a typical dense granulation patch on the external and internal surface of the legs near the claws. However, note that this observation was made on two different specimens (not very well oriented/positioned and stretched on the slide) thus, the certain distribution pattern of body granulation requires a further examination when a new population of T. voronkovi becomes available. As in the original description, the eggs of T. voronkovi have small conical processes with elongated and flexible apices which are often folded and not clearly visible or even broken. ( Figs 21A View Fig , C–J, 22). The process walls are smooth, without any obvious thickenings or tubercles ( Fig. 22 View Fig ) but with obvious annulation and with the labyrinthine layer within process walls, visible under LCM as roundish polygonal reticulation ( Fig. 21C View Fig , E–F), on one egg being abnormally developed and visible more like pores than true reticulation ( Fig. 21D View Fig ). Under SEM, the surface between processes is covered with short irregular striae/ridges/wrinkles which often radiate from the process bases, with small micropores randomly scattered in between them ( Fig. 22 View Fig ). However, under PCM the surface is visible as being covered with dark dots which are probably the thickenings of the labyrinthine layer within the chorion ( Fig. 21B View Fig ).
The morphological analysis conducted on two populations designated as “ T. voronkovi ” by Zawierucha et al. (2016a), from Edgeøya and Nordaustlandet (islands within the Svalbard archipelago, Norway), showed distinct differences in cuticle morphology in comparison to the T. voronkovi type series. Specifically, animals of the Edgeøya population exhibit faint, dense and uniformly distributed granulation on the whole dorso-lateral cuticle from its cephalic to the caudal end (excluding ventral and leg cuticle) ( Fig. 23A View Fig ), whereas this granulation is absent or not visible under LCM in animals of the Nordaustlandet population. The morphology of egg processes in both these populations is very similar: specifically, processes are of a conical shape with elongated apices, with the labyrinthine layer between the process walls clearly visible under LCM as a reticular pattern with sinuous margins and elongated meshes decreasing in size from the base to the processes top in most cases ( Figs 23 View Fig B–D, 24). Other traits are as described by Zawierucha et. al. (2016a) however, it should be noted that as for the similarly to T. voronkovi , no more conclusions can be made based on this material due to the bad condition of the slides, with bubbles of air and crystalized Hoyer which prevent further investigation and limit the number of specimens suitable for imaging.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |