Parateleopus Smith & Radcliffe 1912
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5092.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:7544F20D-D9DF-4E04-AC57-E2637CCB7424 |
|
DOI |
https://doi.org/10.5281/zenodo.5883189 |
|
persistent identifier |
https://treatment.plazi.org/id/17273709-FFF4-9621-9DCD-FC372C9E5CB1 |
|
treatment provided by |
Plazi |
|
scientific name |
Parateleopus Smith & Radcliffe 1912 |
| status |
|
Parateleopus Smith & Radcliffe 1912 View in CoL
Type species. Parateleopus microstomus Smith & Radcliffe 1912: 140 View in CoL (type locality: near Makyan Island in the Moluccas, Indonesia, lat. 0°19′20″N., long. 127°28′30″E.) GoogleMaps
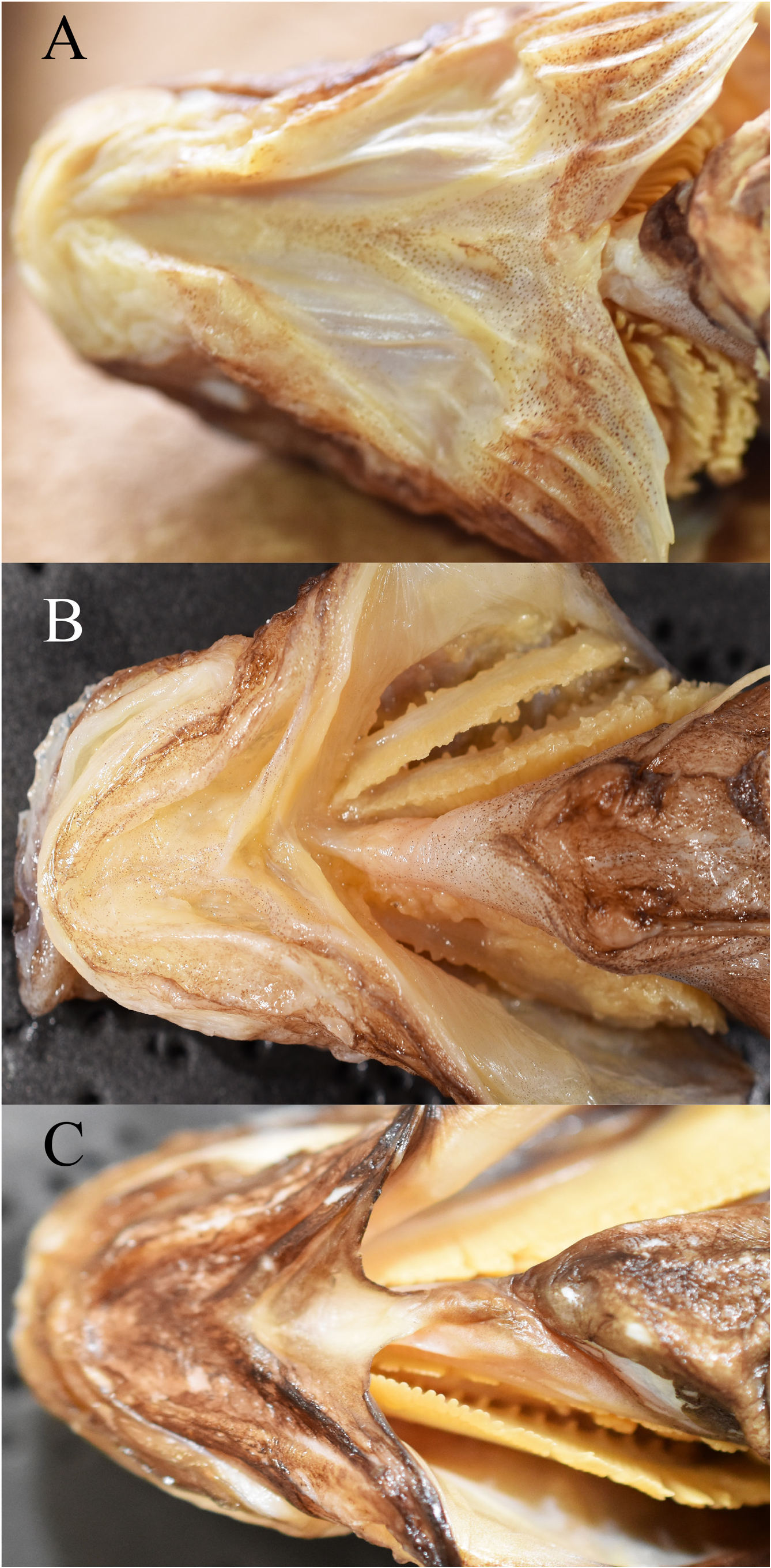
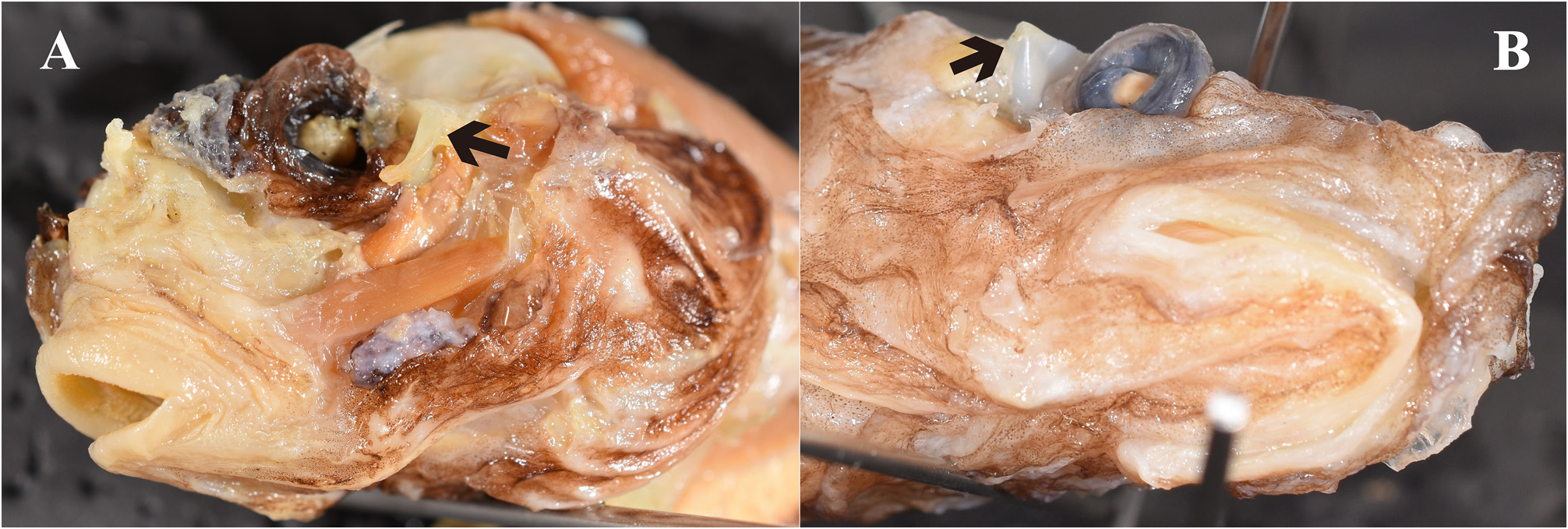
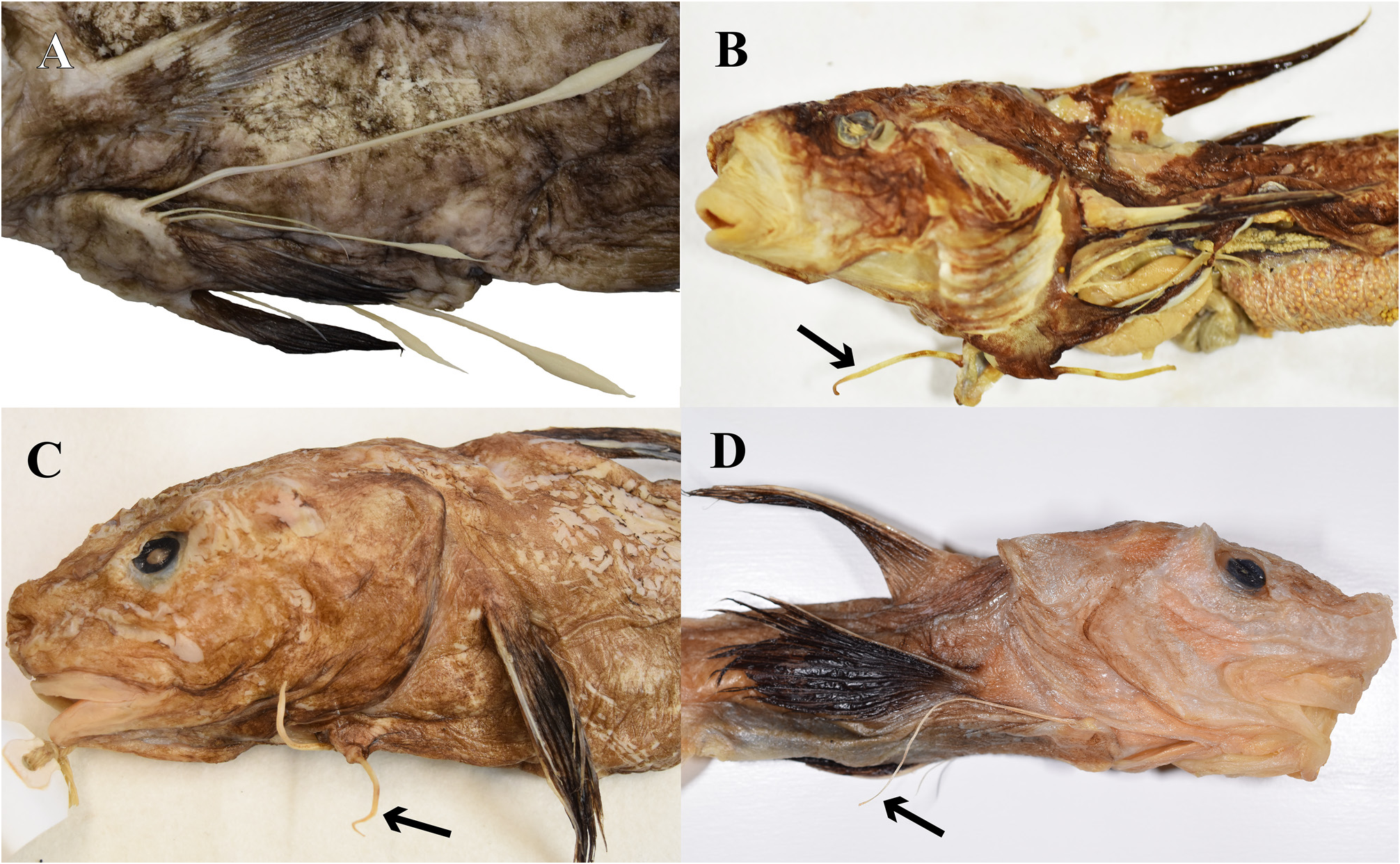
Diagnosis. The following combination of characters distinguishes Parateleopus from other ateleopodid genera: small mouth, posterior end of gape vertical to near anterior nostril ( Fig. 1A View FIGURE 1 ); gill membranes united ventrally and attached to isthmus by soft tissue ( Fig. 2A View FIGURE 2 ); sphenotic has vertically oblong crescent-shaped protrusion from behind eye to posteroinferior region of orbit ( Fig. 3A View FIGURE 3 ); pelvic fin stiff, relatively short, the tip extending to point anywhere between base and anterior half of pectoral fin ( Fig. 4B View FIGURE 4 ); wide pelvic bone ( Fig. 6 View FIGURE 6 ).
Comparisons. Characters distinguishing ateleopodid genera are summarized in Table 1 View TABLE 1 .
Mouth small, the posterior end of gape extending to a little ahead of anterior nostril and one eye diameter anterior from anterior margin of eye in P. indicus ( Fig. 1A View FIGURE 1 ), and “maxillary not reaching vertical to anterior margin of eye” in P. microstomus ( Radcliffe 1912: 140) . In the remaining genera of the family ( Ateleopus , Ijimaia , and Guentherus ), the mouth is large, the posterior end of gape extending to a vertical through anterior margin of eye ( Fig. 1B View FIGURE 1 ). Lower jaw short in P. indicus , the posterior end of lower jaw situated vertical to pupil ( Fig. 1A View FIGURE 1 ), but vertical to posterior margin of eye or beyond in all other genera ( Fig. 1B View FIGURE 1 ). The original description of P. microstomus did not mention the posterior end of gape and lower jaw, but they seem to be situated vertical to anterior nostril and to posterior margin of eye, respectively, in figure 11 in Radcliffe (1912).
Gill membranes united ventrally and attached to the isthmus by soft tissue in P. indicus ( Fig. 2A View FIGURE 2 ). Left and right gill membranes separated and free from isthmus in Ateleopus and Ijimaia ( Fig. 2B View FIGURE 2 ). Gill membranes united to ventrolateral parts of the middle of the isthmus in Guentherus ( Fig. 2C View FIGURE 2 ). The condition in Parateleopus microstomus is unknown.
The sphenotic of P. indicus possesses a vertically oblong crescent-shaped protrusion extending from behind eye to posteroinferior region of orbit ( Fig. 3A View FIGURE 3 ). The sphenotic possesses a sharp spine behind the eye ( Fig. 3B View FIGURE 3 ) in all other genera. Radcliffe (1912: 139) records the sphenotic of P. microstomus as absent: “the strong bony protuberance above and behind the eye is lacking” and “no sharp, knob-like structure above or behind eye” ( Radcliffe 1912: 140).
Parateleopus microstomus has three dorsal-fin rays, a very different number from that in the remaining genera of the family. Our counts were 8–10, 8–12, 10–13, and 9–10 in P. indicus , Ateleopus , Guentherus , and Ijimaia , respectively.
The pelvic fin of Guentherus ( Fig. 4A View FIGURE 4 ) has three free rays followed by normal rays ( Smith 1986; Senou et al. 2008; Kaga 2016; this study). The pelvic fin in the remaining genera appears to be one ray, but it is actually composed of several minute rudimentary rays attached to the front base of this dominant ray and some vestigial rays in the cutaneous membrane on the medial side of the base of that ray ( Kaga et al. 2015: Fig. 3 View FIGURE 3 ). The elongated pelvic ray in P. indicus is stiff and relatively short, with the tip extending to point anywhere between the base of the pectoral fin and the anterior half of that fin ( Fig. 4B View FIGURE 4 ). The elongated ray of Ijimaia is stiff and short, the tip scarcely reaching a vertical through the origin of the pectoral fin or edge of the opercle ( Fig. 4C View FIGURE 4 ), although the pelvic fin in juvenile of Ijimaia extends slightly beyond the origin of the pectoral fin ( Kaga et al. 2015; this study). The pelvic fin of P. indicus resembles that of Ijimaia but may be slightly longer. The pelvic fin of Ateleopus is soft and long, the tip extending to a point anywhere between the base of the pectoral fin and proximity of the anus ( Fig. 4D View FIGURE 4 ; also see Kaga et al. 2015: Fig. 3 View FIGURE 3 ; Kaga 2016). Radcliffe (1912) did not mention the posterior extent of the tip of the pelvic fin and the softness of the elongated ray in Parateleopus microstomus . However, the original figure (op. cit., Fig. 11) of P. microstomus appears to show a relatively short pelvic fin extending to the anterior quarter of the pectoral fin.
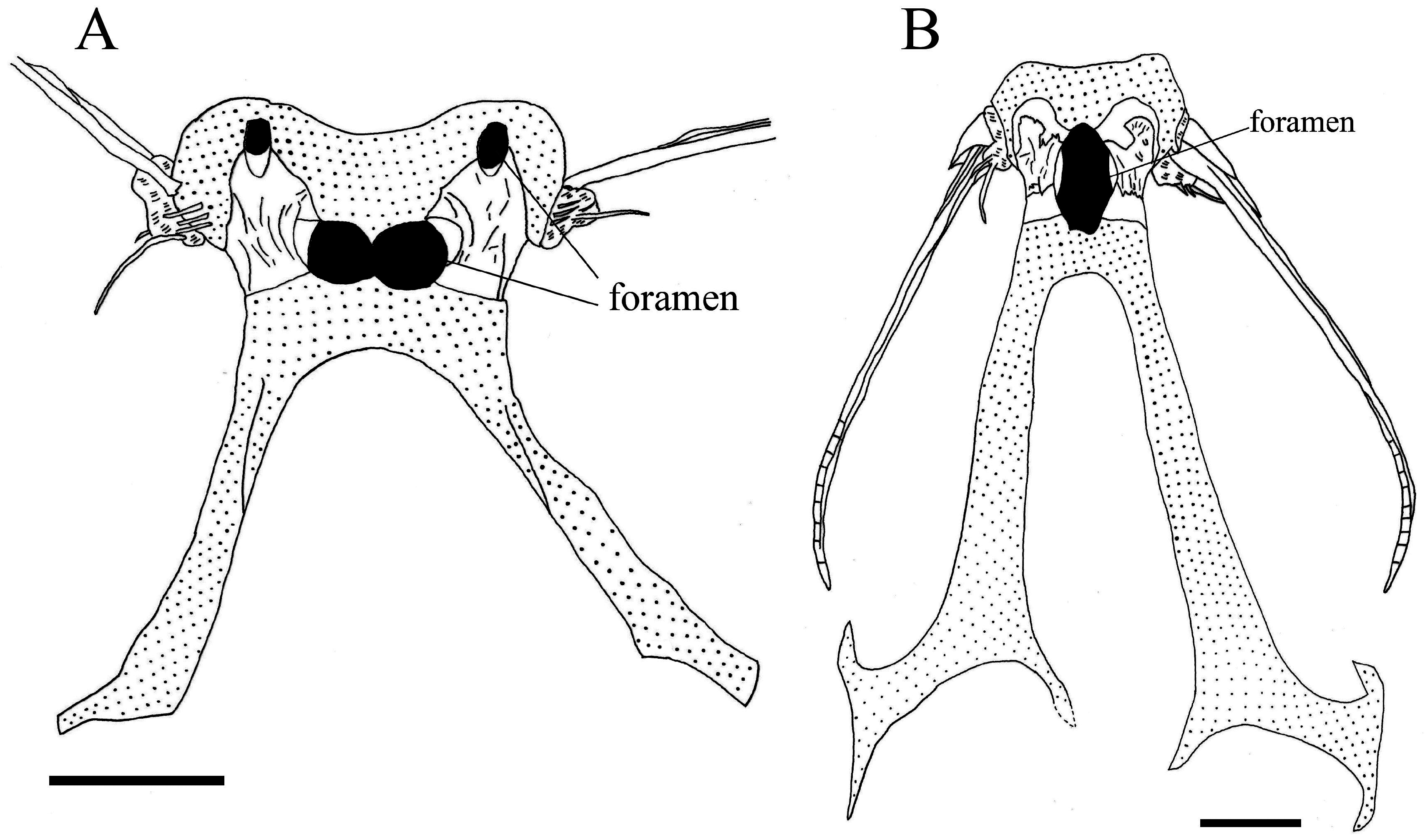
Howell Rivero (1935) described the pelvic bones of Ateleopus and A. indicus as wide, compared to those of Ijimaia . Based on our observation, the pelvic bone of Ijimaia ( Fig. 7B View FIGURE 7 ) is also narrower than those in Ateleopus ( Fig. 7A View FIGURE 7 ) and Parateleopus ( Fig. 6 View FIGURE 6 ). The pelvic bone of Guentherus is also wide ( da Franca & Lorete-Ferreira 1967: Fig. 5 View FIGURE 5 ).
From our study, we have determined the number of vertebrae in P. indicus is 23–25+78–81=101–105. The count is slightly greater in Ateleopus (26–29+81–107=109–136), and much larger in Ijimaia (28–33+96–116=125–149). The number in Guentherus (24–29+50–62=76–91) is much less than in P. indicus and the number is reflected in the length of the tail ( Fig. 5 View FIGURE 5 ABCD), which in Guentherus is much shorter than that of the other genera ( Fig. 5D View FIGURE 5 ). The number of vertebrae in P. microstomus was not recorded by previous investigators and will remain unknown until the holotype is found or other specimens of the species are captured.
Discussion. Parateleopus indicus possesses character states that differ from those of other ateleopodid genera especially in terms of mouth size, gill membrane attachment to isthmus, and protrusion of the sphenotic bone. The species closely resembles Parateleopus microstomus by its small mouth, no sharp spine on sphenotic, and length of elongated pelvic-fin ray. The character states of gill membrane and vertebrae number in P. microstomus cannot be ascertained for want of specimens of that species. The three dorsal-fin rays of Parateleopus distinguish the species from all other ateleopodids. However, it is possibly an abnormality, a result of damage, or a degenerative condition.
The capture locality of P. microstomus is near Makyan Island in Moluccas, Indonesia, which is within the distributional range of P. indicus . The number (91) of anal+caudal-fin rays in P. microstomus (fide Radcliffe 1912) is also within the range (70–92) of P. indicus ( Table 2 View TABLE 2 ). There is a possibility that the two species are synonymous. Unfortunately, the type and only specimen of P. microstomus is lost, and we cannot verify whether or not the small number of dorsal-fin rays is an anomaly. Therefore, we provisionally recognize two species in the genus Parateleopus ( P. microstomus and P. indicus ) until the type specimen of P. microstomus is found or additional specimens of P. microstomus are obtained. That both species have a uniquely (for the family) small mouth and a consequent similarity in their overall head morphology, leads us to infer that they also have a similar gill-membrane attachment to the isthmus. This character can be only seen in the two species among the ateleopodids. Hence, we use the condition of gill membranes united ventrally and attached to the isthmus by soft tissue as another character state defining the genus Parateleopus . We consider the count of anal+caudal-fin rays as another possible diagnostic feature of the genus, since the count in the two species fall within a fairly narrow range. However, because of limited data points and the potential for intraspecific variation, we have eschewed such an action.
Howell Rivero (1935) used the number of foramina in the pelvic bone for classifying the genera in the family Ateleopodidae . He stated that the genus Ijimaia has one foramen in the pelvic girdle (op. cit., Fig. 2 View FIGURE 2 ), and Ateleopus and Parateleopus have two foramina (op. cit., Fig. 1 View FIGURE 1 ). He erected the subfamilies Ateleopinae and Ijimainae diagnosing them by “pelvis wide, with two foramina and two ossified lamina” and “pelvis narrow, with one median foramen and no ossified lamina”, respectively, although no recent researcher has recognized the subfamilies (e.g., Nelson et al. 2016). Howell Rivero noted that Parateleopus has two foramina in the pelvic bone in his key to the genera. He implied that the type specimen of P. microstomus (USNM 72951) had been seen and it was female. He also reported that a specimen of Ateleopus indicus (USNM 98816, 138 mm TL) had been observed (probably by Dr. Ethelwynn Trewavas). The belly of that specimen had already been cut open when we examined it. However, the pelvic bone could not be readily observed for the presence of a foramen. Owing to its small size, it is likely that the foramen had yet to be developed, and damaged to cartilage of the bone rendered further exploration fruitless. Regan’s figure of the pelvic bone in his specimen (1911: Fig. 7B View FIGURE 7 ) shows two small foramina in the pelvic bone, but he did not record them, nor the size of the specimen, or the specimen accession number. We examined two specimens of P. indicus (BMNH 1900.1.20.2, 250 mm SL, and one specimen from lot ZSI F456–457 and 460, 291 mm SL) that had previously opened bellys; the specimens had one wide central foramen in their pelvic bone ( Fig. 6 View FIGURE 6 ). Guentherus has one median foramen on the ventral side of the pelvic bone ( da Franca & Lorete-Ferreira 1967: Figs. 5 View FIGURE 5 and 6 View FIGURE 6 ), but the foramen of the dorsal side opens at the posterior part of the bone ( Barnard 1948: Fig. 5C View FIGURE 5 ). The location of these foramina in Guentherus differs from those of other genera, which are situated at the center of the pelvic bone. From our studies, the number of pelvic-bone foramina in Ateleopus japonicus (295 mm SL) is one at the center and two small foramina at the anterior part of the pelvic bone ( Fig. 7A View FIGURE 7 ). Howell Rivero (1935), using a small specimen of A. japonicus (240 mm TL) reported the foramen number at the center as two. It appears that in Ateleopus the two foramina at the center of the pelvis in small specimens become one foramen with growth. Ijimaia has one foramen at the center of the pelvis ( Fig. 7B View FIGURE 7 ), but may also have two foramina when small, as evidenced by small specimens we examined having the posterior corner of the foramen with vestiges of one convex foramen at the center and two hollows on the sides that likely represent vestiges of two foramina. Our finding of intraspecific variation in the number of the foramina of the pelvic girdle precluded our use of this character in our diagnosis. Howell Rivero (1935) recorded no ossified lamina in Ijimaia , but we observed two such lamina on the pelvis of Ijimaia ( Fig. 7B View FIGURE 7 ).
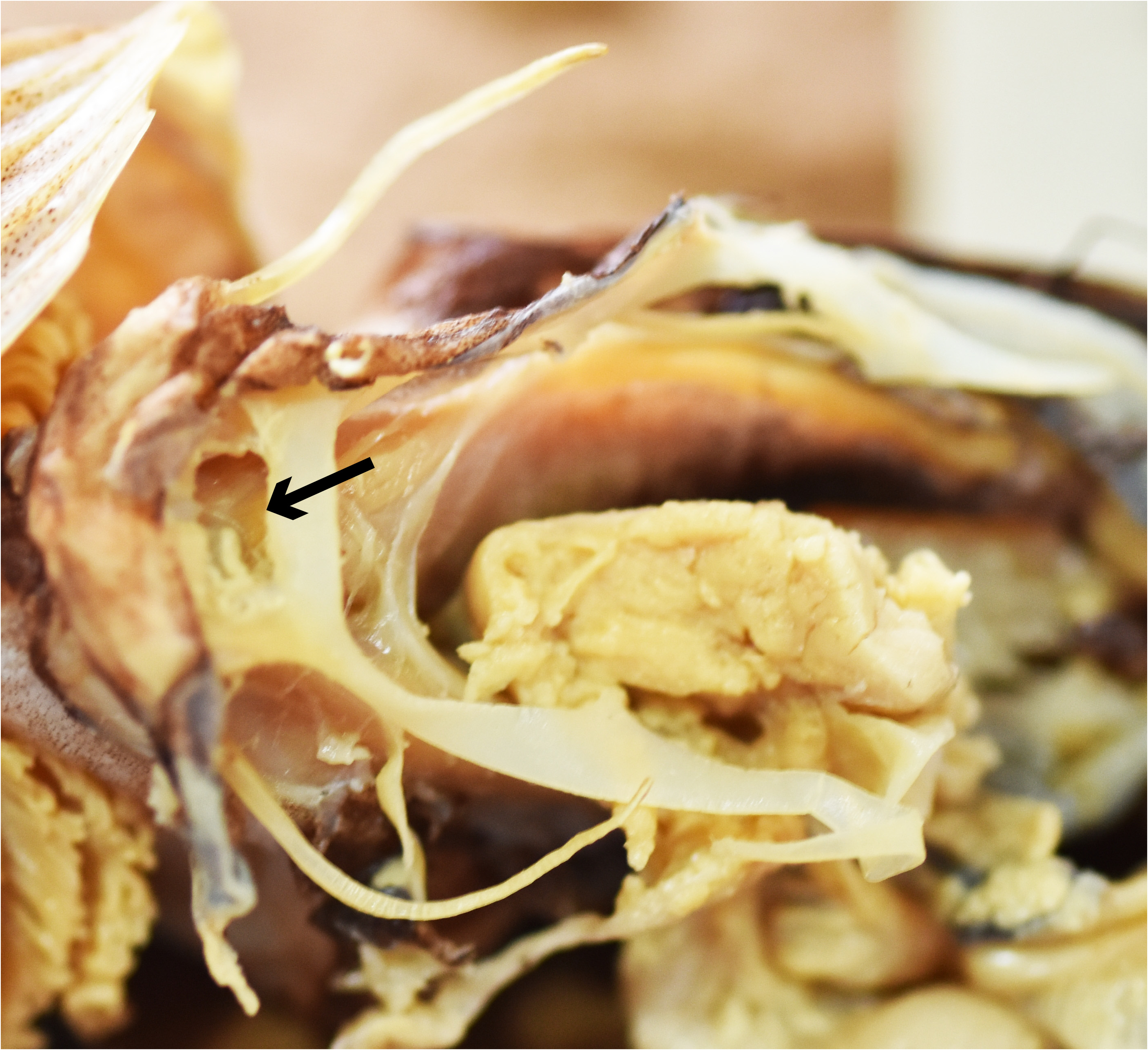
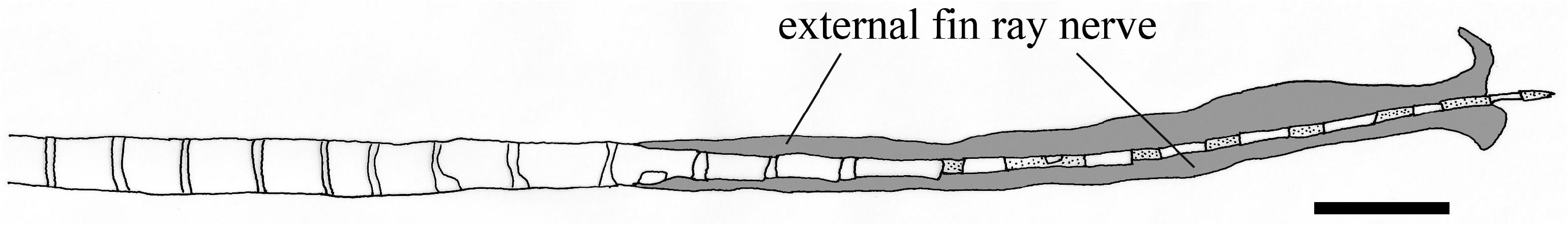
Ecological inference. From our anatomical study, the spinal nerve in Ateleopus innervates approximately seven-eighth the length of the pelvic-fin ray internally, after which it emerges to course externally and completely cover the filamentous tip ( Fig. 8 View FIGURE 8 ) (the external fin-ray nerve was probably broken at the tip of the fin ray in the specimen figured). The same innervation pattern of the spinal nerve in the pelvic-fin ray was reported in fishes of the family Sillaginidae by Kaga (2013: Fig. 84). The sillaginids are engybenthic (sensu Mead 1970) and active foragers in their mode of life, which is near the bottom where they search for benthic organisms using their first soft pelvicfin ray as a sensory receptor ( Kaga 2013).
We observed snails, bivalves, echinoderms (sand dollars and brittle stars), and crustaceans in the stomachs of the Ateleopodiformes . They are all indicators of a benthic feeding mode in these fishes. It is inferred that ateleopodids maintain neutral buoyancy over the deep-sea bottom by way of their gelatinous, watery body and reduced skeletal ossification. They likely use their pelvic-fin ray as a sensory receptor in their search for prey. Ateleopus spp. have a long, soft pelvic-fin ray with a swollen, slightly flattened tip ( Kaga et al. 2015; Kaga 2016; Kaga 2017). This swollen tip is formed by the external fin-ray nerve and the tapered tip of the fin ray. Cartilage was found between the segments of the tip of the pelvic fin in Ateleopus ( Fig. 8 View FIGURE 8 ). The tip of that ray of the Ateleopus is soft, movable and durable. A similar condition was also observed in two of three free rays of the pelvic fin in Guentherus ( Figs. 4A View FIGURE 4 and 5D View FIGURE 5 ). The structure and use of the two free rays in Guentherus are probably the same as in Ateleopus . In contrast, Ijimaia has a short stiff pelvic-fin ray with a tip that appears to lack an external fin-ray nerve and cartilage between segments ( Fig. 7B View FIGURE 7 ), although the spinal nerve does innervate the fin ray. The pelvic-fin ray of Parateleopus resembles that fin ray of Ijimaia . Therefore, it is inferred that Guentherus and Ateleopus use their pelvic-fin rays more for searching benthic animals than do Ijimaia and Parateleopus . But in addition to feeding on benthos, Barnard (1948) reported the probable scales of Photichthys argenteus in the stomachs of Ijimaia loppei , suggesting that the fish may eat live fish or merely scavenge them. We can infer that in their search for food, Ijimaia and Parateleopus use other sensory organs, such as olfactory organ, eye, lateral line, and their sensory canal-bearing bony ossicles in their head ( Sasaki et al. 2006: Fig.1 View FIGURE 1 ). Howell Rivero (1935) recorded the contents of the stomach of I. fowleri as sea urchins, brittle stars, and grains of sand. The sand grains were likely ingested during their feeding on the benthos.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |