Lycalopex vetulus ( Lund, 1842 )
|
publication ID |
https://doi.org/ 10.1644/847.1 |
|
persistent identifier |
https://treatment.plazi.org/id/1A6DE312-FFCE-C639-FF5E-FC4FFC7F6DDB |
|
treatment provided by |
Carolina |
|
scientific name |
Lycalopex vetulus ( Lund, 1842 ) |
| status |
|
Lycalopex vetulus ( Lund, 1842) View in CoL
Hoary Fox
Canis azarae: Lund, 1839:31 . Not Canis azarae Wied- Neuwied, 1824:pl. 23.
Canis vetulus Lund, 1842:4 View in CoL . Type locality ‘‘ Rio das Velhas’s Floddal,’’ Lagoa Santa, Minas Gerais, Brazil.
Canis fulvicaudus Lund, 1843:20 . Type locality ‘‘ Rio das Velhas’s Floddal,’’ Lagoa Santa, Minas Gerais, Brazil.
Vulpes vetulus: Gerrard, 1862:88 . Name combination.
[ Lycalopex fulvicaudus ] var. chilensis Gray, 1869:511 . Type locality ‘‘ South America. ’’
Canis parvidens Mivart, 1890:76 . Type locality ‘‘ Brazil.’’
Canis urostictus Mivart, 1890:81 . Type locality ‘‘ Brazil.’’
[ Canis (Thous) ] parvidens: Trouessart, 1897:308 . Name combination.
[ Canis (Thous) ] urostictus: Trouessart, 1897:308 . Name combination.
Nothocyon urostictus: Wortman and Matthew, 1899:125 . Name combination.
Nothocyon parvidens: Wortman and Matthew, 1899:126 . Name combination.
Canis sladeni Thomas, 1904:235 . Type locality ‘‘ Santa Anna de Chapada [5 Chapada dos Guimarães; latitude 15 u 259S, longitude 55 u 479W],’’ Mato Grosso, Brazil.
[ Canis (Nothocyon) ] parvidens: Trouessart, 1904:235 . Name combination.
[ Canis (Nothocyon) ] urostictus: Trouessart, 1904:235 . Name combination.
E [unothocyon]. sladeni: J. A. Allen, 1905:152 , footnote. Name combination.
E [unothocyon]. urostictus: J. A. Allen, 1905:152 , footnote. Name combination.
E [unothocyon]. parvidens: J. A. Allen, 1905:152 , footnote. Name combination.
Canis (Eunothocyon) vetulus: Ihering, 1911:206 View in CoL . Name combination.
Canis vitulus Huber, 1925:1 . Incorrect subsequent spelling of Canis vetulus Lund, 1842 View in CoL .
Lycalopex vetulus: Kraglievich, 1930:43 View in CoL . First use of current name combination.
Lycalopex vetulus fulvicaudus: Kraglievich, 1930:43 . Name combination.
Dusicyon (Lycalopex) vetulus: Osgood, 1934:49 . Name combination.
P [seudalopex]. vetulus: Berta, 1987:458 . Name combination.
CONTEXT AND CONTENT. Order Carnivora , suborder Caniformia , family Canidae , subfamily Caninae . No subspecies are currently recognized ( Stains 1975; Wozencraft 2005). Synonymy is modified from Zunino et al. 1995.
NOMENCLATURAL NOTES. Burmeister (1854) created the genus Lycalopex for Pseudalopex vetulus , subsequently followed by Gray (1869). Osgood (1934) reduced Lycalopex to a subgenus of Dusicyon ; considered a subgenus of Dusicyon by Cabrera (1958). Langguth (1975) put the species in a separate genus, Lycalopex vetulus . Included in Dusicyon by Clutton-Brock et al. (1976); in Canis (Lycalopex) by Van Gelder (1978); placed in Pseudalopex by Berta (1987), and followed by Wozencraft (1993). Wozencraft (2005) assigned vetulus to Lycalopex .
Other names for the hoary fox are hoary zorro, smalltoothed dog (English), renard du Bresil (French), kampfuchs ( Germany), raposa-do-campo, and raposinha (Portuguese). In the indigenous language of Brazil, L. vetulus is called jaguarapitanga (Tupy) and waptsã wa (Xavante).
DIAGNOSIS
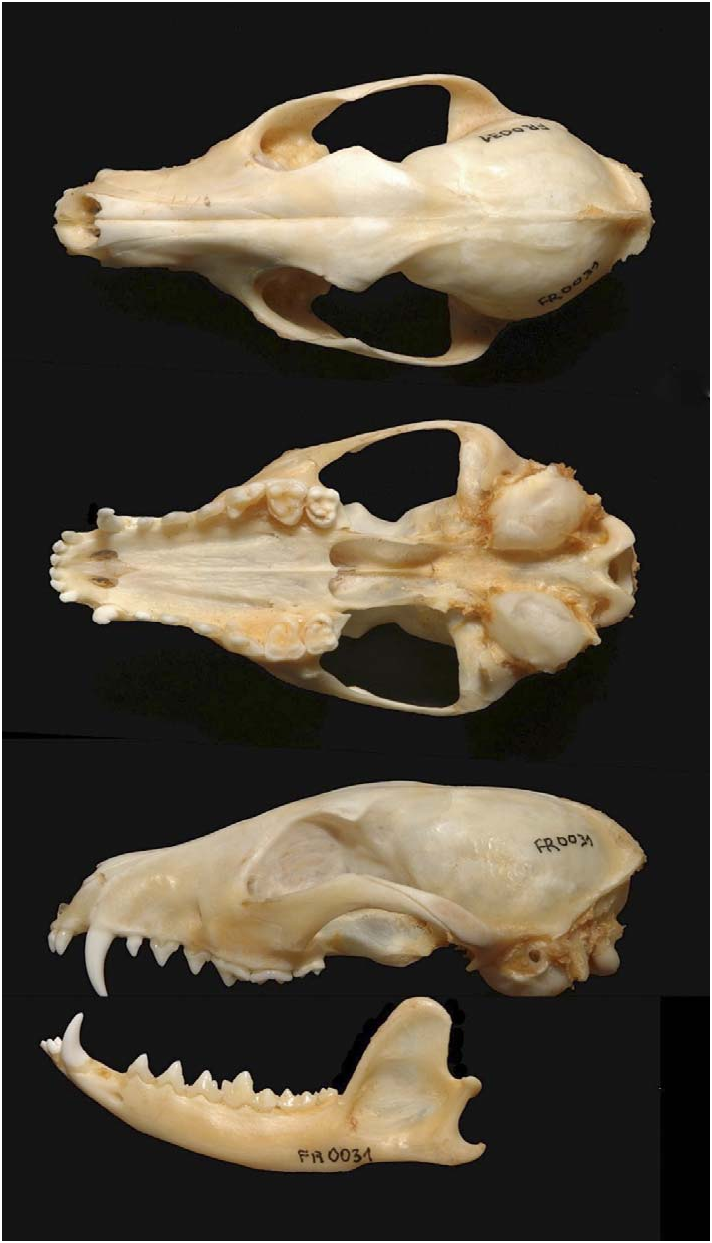
Lycalopex vetulus ( Fig. 1 View Fig ) differs from other South American foxes ( Cerdocyon thous [crab-eating fox], Dusicyon australis [ Falkland Islands fox], L. culpaeus [culpeo], L. fulvipes [Darwin’s fox], L. griseus [South American gray fox], L. gymnocercus [pampas fox], and L. sechurae [sechuran fox]) by its short muzzle, small skull (faciocephalic index about 45), and reduced carnassials. For L. vetulus , the length of P4 is slightly greater than length of M1, length of m1 is about equal to the length of m2 + m3, and upper molars are subquadrate with very little transverse extension ( Fig. 2 View Fig ; Cabrera 1931). Analysis of phenetic characters aligns L. vetulus very closely to the sechuran fox (Clutton-Brock et al. 1976). L. vetulus differs from C. thous azarae (a subspecies of the crab-eating fox from Brazilian northeastern caatinga) in having a shorter muzzle, thicker fur, and rust-yellow ears and legs ( Vieira 1946). Teeth of L. vetulus are much smaller than those in Cerdocyon and differ from those of Cerdocyon in the following characters: incisors more strongly tricuspidate; upper and lower canines shorter, and upper molars transversally more straight; 3rd lower premolar shows a single salience in posterior edge (de Paula-Couto 1950). The neck of L. vetulus is relatively longer and thinner than that of the crab-eating fox, whose cervical vertebrae are much larger; lumbar vertebrae of L. vetulus are strongly narrow and thin; articulations are longer and thinner than those of the crab-eating fox. Structure and proportions of long tubular bones of limbs differ between the 2 species: bones of both species are exactly the same length, but thickness of L. vetulus bones is not more than one-half that of the crabeating fox (de Paula-Couto 1950).
GENERAL CHARACTERS
Lycalopex vetulus is a gracile animal, with a small head, and slender body and limbs. Upper parts of body are pale gray and underparts are yellow. Anterior part of neck is white and the chest and the remainder of the neck are yellow-brown. Lower jaw and the tip and superior base of tail are black; a buff-yellow patch occurs behind ear (de Paula-Couto 1950). Pelage contains a mixture of short, woolly, thin, rough fur and long, hard, erect fur (de Paula- Couto 1950). In both sexes, general color is yellowish gray, although males may have a strip of black hair from nape to end of tail ( Vieira 1946). Near-melanistic forms of L. vetulus are found with some frequency ( Cabrera 1931; Cabrera and Yepes 1960; Vieira 1946). Two blackish L. vetulus from the same litter were sighted in November 1995, about 4 km north of Nova Xavantina, eastern Mato Grosso ( Dalponte 2003).
Lycalopex vetulus is the smallest Brazilian canid. Mass is about 3–4 kg (Dalponte and Courtenay 2004; Nowak and Paradiso 1983). Body measurements (mm) for mixed sex, adult L. vetulus (de Paula-Couto 1950; Nowak and Paradiso 1983; Vieira 1946) were: length of head and body, 580–715 (n 5 10); length of tail, 250–355 (n 5 11); length of ear, 60–75 (n 5 7); height at shoulder, 327–375 (n 5 2); length of hind foot, 120–135 (n 5 6). Mean cranial dimensions (mm; ranges in parentheses) for 4 adults ( Vieira 1946) were: total length of skull, 114.7 (111–118); bizygomatic width, 63.7 (61–68); cranial width, 41.7 (41–42); interorbital width, 19 (16–20); length of upper toothrow, 38.2 (37–39); length of mandible, 84 (82–86); palatal length, 54.5 (52–59). Tubercular molars of L. vetulus are proportionately larger than those in any living canid; canines are sharply pointed and foxlike. L. vetulus has a very narrow lyriform sagittal area and a slight interparietal crest; anterior part of frontal bone is slightly swollen ( Fig. 2 View Fig ; Clutton-Brock et al. 1976; Osgood 1934; Stains 1975).
DISTRIBUTION
Lycalopex vetulus is endemic to Brazil and occurs mainly in the south-central Brazilian cerrados (a savannalike vegetation) at an elevational range of 90–1,100 m. Its geographic range extends west of São Paulo State north to at least Piauí State, through the states of Mato Grosso do Sul, Mato Grosso, Goiás, Minas Gerais, Tocantins, Bahia, and probably open areas of the southern regions of Maranhão and Rondônia states ( Fig. 3 View Fig ; Barbosa Souza and Olmos 1991; Cabrera 1931; Carvalho 1980; Coimbra- Filho 1977; Costa and Courtenay 2003; Dalponte 2003; de Paula-Couto 1950; Langguth 1975; Santos 1945). Occurrence in Ceará State, as reported by Deane (1956), is contested by Courtenay et al. (1996). Although Paraná State has been suggested as the southernmost distribution limit for the species ( Vieira 1946), there is no strong evidence to support this possibility. L. vetulus is replaced in Paraná and areas to the south by the pampas fox ( Cabrera 1958). L. vetulus is thought to occur in Bolivia, in the Huanchaca area ( Anderson 1997).
FOSSIL RECORD
Lycalopex vetulus is known from the Lujanian mammal age (late Pleistocene, 300,000 to 10,000 years ago) of Argentina and Brazil ( Berta 1987). Fossils of L. vetulus were recovered during P. Lund’s excavations in the Lagoa Santa Caves, Minas Gerais, Brazil (de Paula-Couto 1950; Winge 1895).
FORM AND FUNCTION
Lycalopex vetulus is categorized as a small canid ( Moehlman 1989). Its lightly built extremities, elongated only in the metapodia, and peculiar dentition, large molars and very small carnassials ( Langguth 1969), suggest special adaptations quite different from those of small canids of the Patagonian subregion ( Langguth 1975). Small carnassial apparatus and wide crushing molars of L. vetulus suggest an insectivorous diet rather than one of large vertebrates ( Langguth 1975). The small skull composed of relatively fragile bones, with a short muzzle and a salient sagittal crest present only in adult males ( Vieira 1946), also is consistent with a diet based on small items. Dental formula is i 3/3, c 1/ 1, p 4/4, m 2/3, total 42. Auditory bullae of L. vetulus are larger than those of other South American canids (Clutton- Brock et al. 1976; Ihering 1911), possibly indicating increased auditory ability to detect foraging harvester termites ( Syntermes and Cornitermes ).
Many morphological features of L. vetulus , including small size, thin body, tall and slender limbs, and long tail, represent adaptations for travelling and searching for food in the underbrush of grasslands ( Dalponte 1997). L. vetulus is agile and swift, and during escape play can jump and run in a zigzag pattern with extreme ability, reducing body contact with vegetation ( Dalponte 1997). Complexity and size of caecum have been suggested as significant features to understanding phylogenetic relationships among Brazilian canids; however, morphology of caecum in L. vetulus is undescribed ( Langguth 1975).
ONTOGENY AND REPRODUCTION
Births occur in spring, with usually 2 or 4 young per litter ( Bueler 1973; Grzimek 1975). At Rio de Janeiro Zoo, young were born in early September and litter size was 4 (Coimbra-Filho 1966). At Cuiabá Zoo, Cuiabá, Brazil, young were born in mid-August and litter size was 3 ( Dalponte 2003). Several young about 3 weeks old were obtained in mid-October by P. Lund in Lagoa Santa, Minas Gerais (de Paula-Couto 1950). Lactating females were observed in September in eastern Mato Grosso ( Dalponte 2003) and in October in southern Bahia, Brazil (Juarez and Marinho-Filho 2002). In a recent field study in Mato Grosso ( Dalponte 2003), mating occurred in June and parturition in August, after a gestation period of about 50 days. A single female produced a litter with 5 young one year and at least 3 young the next year. On both occasions, the female used underground burrows dug by the six-banded armadillo ( Euphractus sexcinctus ) as her den ( Dalponte 2003).
Females nurse their offspring for about 4 months, but may remain with them for an additional 2 months. Barks used by the female during the nursing period were associated with aggressive behavior toward humans and domestic dogs approaching the den area and young ( Dalponte 2003). Dispersion of young occurs about April.
ECOLOGY
Lycalopex vetulus is chiefly active at night. Observations of a free-ranging family group in Nova Xavantina, eastern Mato Grosso State, indicated that activity began after sunset and ended at dawn ( Dalponte 2003). This activity pattern has been confirmed by following radiocollared animals ( Courtenay et al. 2006; Juarez 1997).
Lycalopex vetulus is omnivorous, with insects (particularly harvester termite soldiers and workers, dung beetles, and grasshoppers) and fruits well represented in its diet ( Dalponte 1997). Other arthropods, small mammals, birds, and reptiles are less commonly consumed. Harvester termites and small mammals are consumed more frequently in the dry season; fruits and other insects are consumed more frequently in the wet season ( Dalponte 1997). Studies in other central Brazilian areas corroborate this feeding pattern ( Courtenay et al. 2006; Ferreira-Silva and de Souza Lima 2006; Juarez and Marinho-Filho 2002; Silveira 1999). Syntermes insidians (Termitidae) , a harvester termite associated with the very poor sandy soils of the cerrado ( Constantino 1995), is the main prey of L. vetulus in Chapada dos Guimarães, Mato Grosso State ( Dalponte 1997). The foraging period of L. vetulus overlaps that of S. insidians ( Dalponte 1997) .
Lycalopex vetulus is broadly tolerant of a variety of open habitats, but appears to be most common in cerrado vegetation (e.g., campo cerrado, campo limpo, campo sujo, and cerrado stricto sensu) on smooth highlands and peneplains of central Brazil ( Dalponte 1997; de Paula-Couto 1950; Juarez and Marinho-Filho 2002; Langguth 1975; Silveira 1999). Family groups may use peri-urban landscapes ( Dalponte 2003). L. vetulus is reportedly common in some xeric formations of northeastern Brazil such as the typical sertão ( Deane 1956; Deane and Deane 1954), and in peripheral areas of the caatinga (Barbosa Souza and Olmos 1991). Occurrence of L. vetulus in the central core of caatinga is debated ( Courtenay et al. 1996). It occurs in the relatively dry Nhecolândia region of the Pantanal wetland, but seems to use moist areas less than sympatric canids, such as the crab-eating fox and the maned wolf ( Chrysocyon brachyurus — Dalponte 1995). In southern Bahia, 1 adult female of L. vetulus monitored by radiotelemetry during a 5- month period occupied an area of 385 ha (Juarez and Marinho-Filho 2002). One group (an adult breeding pair and 5 juvenile offspring) living in a cattle pasture in Minas Gerais shared overlapping home ranges of 456 ha ( Courtenay et al. 2006). In pastures of eastern Mato Grosso, 3 study groups comprised a family group (2 females and 2 males) and 2 breeding pairs occupied a mean home range of 48 ha (range 19–149 ha— Dalponte 2003).
In cattle pastures, L. vetulus spent much of the time searching and lapping up harvester termites ( Syntermes and Cornitermes ) from the surface of the ground in the dry season, and scratching fresh cattle dung for beetles in the wet season. Although L. vetulus does not forage cooperatively, members of a family will forage on termites while in close physical proximity. Groups consisting of a single female and her offspring, adult pairs during breeding season, and single foxes have been observed in Mato Grosso. Young L. vetulus , prior to dispersal, and captive individuals show several interactive behaviors, including chasing one another in a zigzag pattern, play fighting with lateral body impacts, and head movements ( Dalponte 2003).
Lycalopex vetulus has been considered the typical grassland canid in central Brazilian cerrado ( Langguth 1975), where its activities result in dispersal of fruits and grasses (Dalponte and Lima 1999; Silberbauer-Gottsberger 1984). Morphological adaptations of L. vetulus for living in dry, open grasslands and a termite-based diet may allow segregation of space and food resources with sympatric canids ( Dalponte 1997). Diet of L. vetulus overlaps little with diets of other sympatric canids and differences between diets were critical to ecological separation among L. vetulus , crabeating fox, and the maned wolf (Juarez and Marinho-Filho 2002; Silveira 1999). Remains of L. vetulus have been found in scats of maned wolves, in central Brazil ( Silveira 1999).
In Ceará State, Northeast Brazil, L. vetulus is regarded as a wild reservoir of American visceral leishmaniasis ( Barros et al. 1989; Deane 1956). However, the crab-eating fox is considered the single wild canid reservoir of American visceral leishmaniasis in Ceará State ( Courtenay et al. 1996). American visceral leishmaniasis is a protozoal zoonosis caused by Leishmania chagasi (5 donovani) transmitted by the sandfly ( Lutzomyia longipalpis — Grimaldi et al. 1989), and maintained in the wild among L. vetulus and the crabeating fox from which peridomestic foci of canine and human visceral leishmaniasis might be derived ( Lainson et al. 1990). Cases of mortality due to sarcoptic mange have been reported for L. vetulus in central Brazil (Serra da Canastra National Park, Minas Gerais State —J. Dietz, in litt.; southern Bahia —J. Marinho-Filho, pers. comm.). Other parasites of L. vetulus include Angiostrongylus vasorum and Trypanosoma cruzi (see references in Dalponte and Courtenay 2004).
GENETICS
Lycalopex vetulus has a diploid number (2n) of 74, and a fundamental number (FN) of 76. There are 36 pairs of acrocentrics and a large metacentric pair. Identification of the X chromosome is uncertain, because the specimen studied was female; as for other members of Canidae , the X is presumed to be a metacentric (Wurster and Benirschke 1968). There is disagreement regarding the genetic relationships of South American canids. Based on karyological information, L. vetulus appears most closely related to Atelocynus , Canis , Chrysocyon , Lycaon , and Speothos ( Chiarelli 1975) . However, Lycalopex , Speothos , and Cerdocyon all have similar karyotypes that differ slightly from the maned wolf and wolf-like canids ( Wayne et al. 1987). Morphological and karyological studies suggest close association between the Dusicyon group (in this case including L. vetulus ) and the crab-eating fox ( Berta 1987; Wayne et al. 1989).
CONSERVATION
Currently, Lycalopex vetulus is widespread and abundant in the highland cerrado of Brazil and there appear to be no known threats that would result in a significant decline in the population (Dalponte and Courtenay 2008). L. vetulus is classified as ‘‘Least Concern’’ by the International Union for Conservation of Nature and Natural Resources (2009). The Canid Conservation Assessment and Management Plan recommended conducting surveys and collecting basic ecological information on L. vetulus (World Conservation Union/Species Survival Commission/Captive Breeding Specialist Group 1994). L. vetulus was categorized as ‘‘vulnerable’’ and in need of a captive program (level 2) by the Mace–Lande criteria for threat (World Conservation Union/ Species Survival Commission/Captive Breeding Specialist Group 1994), and is not listed by Convention on International Trade in Endangered Species (Dalponte and Courtenay 2004).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lycalopex vetulus ( Lund, 1842 )
| Dalponte, Julio C. 2009 |
Dusicyon (Lycalopex) vetulus:
| OSGOOD, W 1934: 49 |
Lycalopex vetulus: Kraglievich, 1930:43
| KRAGLIEVICH, J 1930: 43 |
Lycalopex vetulus fulvicaudus:
| KRAGLIEVICH, J 1930: 43 |
Canis vitulus
| HUBER, A 1925: 1 |
Canis (Eunothocyon) vetulus:
| IHERING, H 1911: 206 |
Canis (Nothocyon)
| TROUESSART, E 1904: 235 |
Canis (Nothocyon)
| TROUESSART, E 1904: 235 |
Canis (Thous)
| TROUESSART, E 1897: 308 |
Canis (Thous)
| TROUESSART, E 1897: 308 |
Canis parvidens
| MIVART, St 1890: 76 |
Canis urostictus
| MIVART, St 1890: 81 |
Lycalopex fulvicaudus
| GRAY, J 1869: 511 |
Vulpes vetulus:
| GERRARD, E 1862: 88 |
Canis fulvicaudus
| LUND, P 1843: 20 |
Canis vetulus
| LUND, P 1842: 4 |
Canis azarae: Lund, 1839:31
| LUND, P 1839: 31 |