Pyronotanthias, Gill, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5092.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:A546CCCB-6072-434B-B366-1AFB1BE20CD8 |
|
DOI |
https://doi.org/10.5281/zenodo.5883171 |
|
persistent identifier |
https://treatment.plazi.org/id/1B7A87C4-FFA3-FFDE-B7AB-1E36FC52FD12 |
|
treatment provided by |
Plazi |
|
scientific name |
Pyronotanthias |
| status |
gen. nov. |
Mirolabrichthys + Nemanthias + Pyronotanthias + Anatolanthias + Luzonichthys + Rabaulichthy s + Tosana
+ Pseudanthias (in part) + Hemanthias + Choranthias . This relationship is supported by a single synapomorphy.
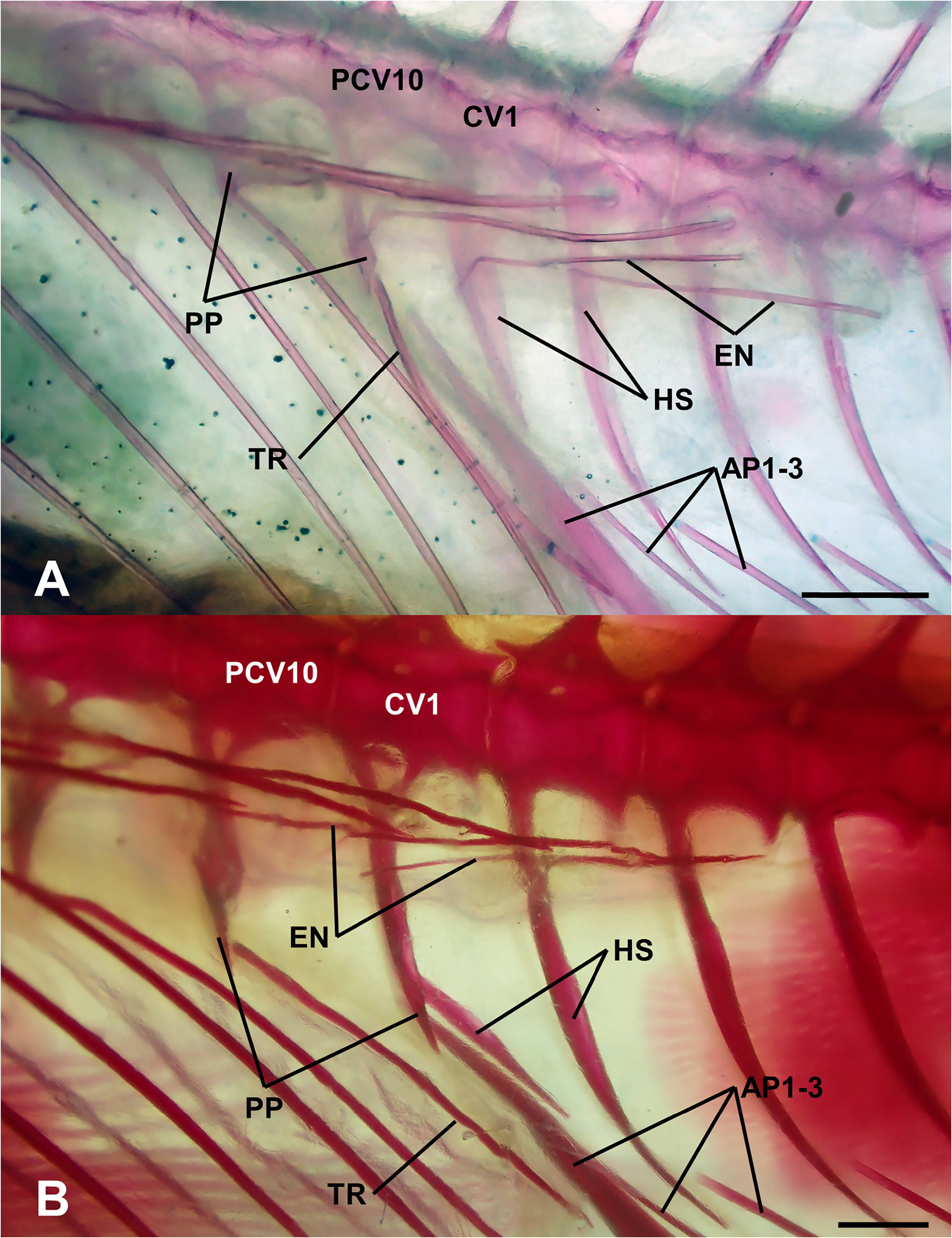
Parapophyses on first caudal vertebrae. Plesiomorphically in anthiadine fishes, the terminal pair of parapophyses are on the terminal precaudal vertebra, which is identified from the adjacent first caudal vertebra in lacking a haemal spine ( Fig. 7A View FIGURE 7 ). In contrast, the first caudal vertebra bears a pair of parapophyses in Mirolabrichthys ( Fig. 7B View FIGURE 7 ), Nemanthias, Pyronotanthias , Anatolanthias , Luzonichthys , Rabaulichthys , Tosana , Hemanthias , Choranthias Anderson & Heemstra, 2012 and all but three species of Pseudanthias : P. hawaiiensis ( Randall, 1979) , P. ventralis ( Randall, 1979) and P. hangapiko Shepherd, Pinheiro, Phelps, Pérez-Matus & Rocha, 2021 . Preliminary molecular studies support exclusion of these three species from Pseudanthias ( Gill et al. 2021a; L. Rocha, pers. comm.). This character was first noted by Baldwin (1990) and briefly reviewed by Pogonoski & Gill (2021).
Pyronotanthias + Nemanthias + Anatolanthias + Luzonichthys + Rabaulichthys . A single character supports this relationship.
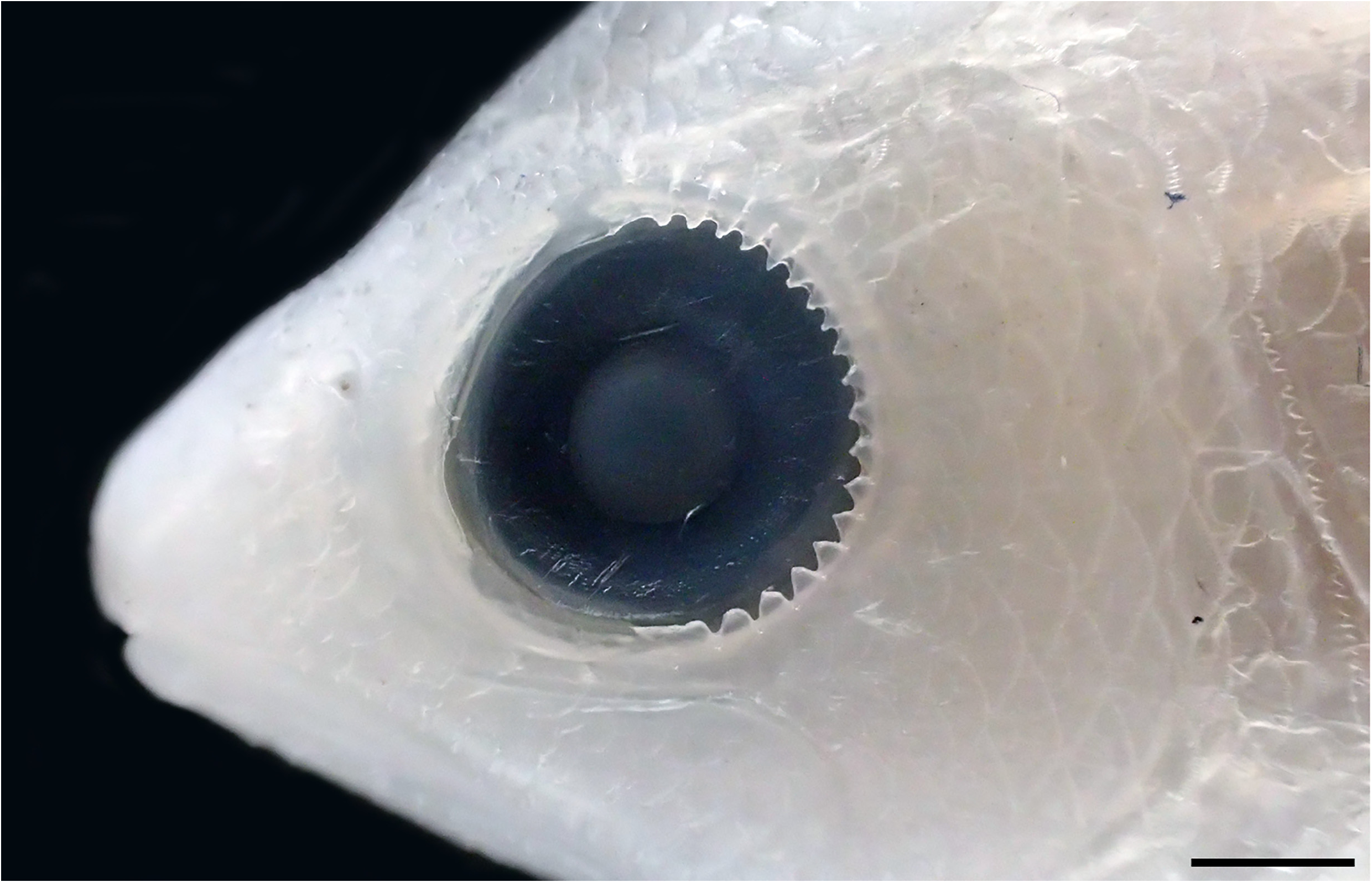
Longest few pectoral-fin rays bearing serrated projections. W.F. Smith-Vaniz (in Randall & Lubbock 1981) noted that Pyronotanthias lori and P. smithvanizi have distinctive serrations on the distal parts of the longest few pectoral-fin rays. Randall & Pyle (2001) subsequently recorded this character in P. privitera ( Randall & Pyle, 2001) . This character also occurs in all examined species of Pyronotanthias . It otherwise occurs in all examined species of
Nemanthias View in CoL ( Fig. 9 View FIGURE 9 ), Anatolanthias View in CoL , Luzonichthys View in CoL and Rabaulichthys View in CoL . However, this character may be more widely distributed, as it is possibly present in Pseudanthias kashiwae ( Tanaka, 1918) View in CoL (other related species in the P. cooperi View in CoL complex not examined; A. mooreanus Herre, 1935 is likely a junior synonym of P. kashiwae View in CoL ). In P. kashiwae View in CoL , some of the longest few pectoral-fin rays have ridge-like expansions dorsally or ventrally, which sometimes bear a few weak serrations.
Nemanthias View in CoL + Anatolanthias View in CoL + Luzonichthys View in CoL + Rabaulichthys View in CoL . Two characters support this relationship.
High number of lateral-line scales. In their key to species of the subgenus Mirolabrichthys, Randall & Lubbock (1981) differentiated the five species here newly incorporated in Nemanthias from other species in the subgenus based on their relative high numbers of lateral-line scales and pectoral-fin rays (53–64 versus 41–56 and 18–22, rarely 18 versus 15–19, rarely 19, respectively). However, N. carberryi has an intermediate number of lateralline scales (50–56). Nonetheless, the counts for N. carberryi are relatively high for anthiadines. Among genera that appear closely related (e.g. with parapophyses on the first caudal vertebra), such high counts are found only in several species of Pseudanthias (notably P. caudalis Kamohara & Katayama, 1959 and P. thompsoni , which appear to be closely related to each other, as well as P. calloura Ida & Sakaue, 2001 (51–53 scales) and P. cooperi and relatives (collectively with 46–55 scales; Randall & Pyle, 2001)), and in species of Anatolanthias (62–64 lateral-line scales; gill arch characters not yet confirmed for the genus), Luzonichthys (51–78 lateral-line scales) and Rabaulichthys (51–59 lateral-line scales).
High number of epineural bones. Anderson et al. (1990) had previously suggested a close relationship between Anatolanthias , Luzonichthys and Rabaulichthys on the basis of several derived features: anterior and posterior nares well separated from each other; vomerine dentition reduced or absent; sum of numbers of pairs of epipleural and epicaudal ribs 16–19. I identify the last-mentioned collectively as epineural bones, following the terminology of Johnson & Patterson (1993, 2001) and Patterson & Johnson (1995). Except for N. bicolor with 12 epineurals, Nemanthias also has relatively high numbers of epineurals (15–17; Table 4 View TABLE 4 ).
Conflicting characters. The following characters have been used to diagnose Mirolabrichthys in the broad sense, or to suggest relationships within the genus. I here consider them to be homoplastic but acknowledge that they suggest relationships or generic classifications alternative to those proposed herein.
Hypertrophied upper lip of males. Mirolabrichthys has been traditionally diagnosed by the presence in males of a hypertrophied upper lip. Randall & Lubbock (1981) noted that similar morphology was also found in Nemanthias carberryi . Hypertrophy of the upper lip is characteristic of all species included here in Mirolabrichthys , Nemanthias and Pyronotanthias , although it is variable in degree depending on species ( Figs. 1 View FIGURE 1 , 8 View FIGURE 8 , 10 View FIGURE 10 ). Similar morphology is not known in Anatolanthias , Luzonichthys or Rabaulichthys , which suggests conflict with the relationships implied by the above characters. Males of Pseudanthias flavicauda Randall & Pyle, 2001 , P. pulcherrimus ( Heemstra & Randall, 1986) , P. randalli ( Lubbock & Allen, 1978) and P. tequila Gill, Tea & Senou, 2017 , have variously developed hypertrophy of the upper lip, which suggests a closer relationship of Mirolabrichthys , Nemanthias and Pyronotanthias with these species (and presumably also P. oumati Williams, Delrieu-Trottin & Planes, 2013 , for which males are unknown, but which groups with P. randalli and P. pulcherrimus in analyses of COI sequences; Williams et al. 2013).
Papillae on orbital rim. Two species of Mirolabrichthys and all species of Pyronotanthias are distinctive in having large fleshy papillae on the posterior part of the orbit ( Fig. 4 View FIGURE 4 ). They are not present in M. evansi or in species of Nemanthias . Among anthiadines, similar orbital papillae are found in species of Anatolanthias , Luzonichthys and Rabaulanthias, as well as one species of Pseudanthias , P. calloura . The character therefore implies relationships and a generic classification counter to the current proposal. However, the character is difficult to interpret in some specimens, and is possibly present in P. kashiwae , in which weakly developed papillae-like projections are apparent in some specimens. Moreover, fleshy orbital papillae are also present in the planktivorous pomacentrids Lepidozygus tapeinosoma ( Bleeker, 1856) , Chromis pamae Randall & McCosker, 1992a and C. randalli Greenfield & Hensley, 1970 , in the serranine serranid genus Schultzea Woods, 1958 and in the anomalopid genus Anomalops Kner, 1868 . According to a recent phylogenetic study, the two species of Chromis are sister taxa, which are only distantly related to Lepidozygus ( Tang et al. 2021) . Johnson & Rosenblatt (1988) suggested the papillae may be associated with maintaining laminar flow of water over the eyeball. Similarly, Randall & McCosker noted the occurrence of orbital papillae in the three pomacentrids and certain anthiadines and suggested the following: “All of these fishes are slender-bodied; they feed on zooplankton well above the substratum but must swim swiftly to cover with the approach of predaceous fishes. We believe the papillae may function to ensure a smooth flow of water over the eye when swimming rapidly” ( Randall & McCosker 1992a: 333). If these explanations are correct, orbital papillae might be expected to occur more widely in anthiadines, as well as perhaps in other taxa with similar behaviour and general morphology such as symphysanodontids, but this is apparently not the case.
Supraneural number and anterior dorsal-fin pterygiophore formula. Plesiomorphically in anthiadines, there are three supraneural bones, arranged in an ADPF of S/S+S/3/1+1. Departures from this condition include loss of one or more supraneurals, and anterior migration of dorsal-fin pterygiophores (autapomorphic for N. carberryi ). Of the three genera considered here, only Mirolabrichthys has the plesiomorphic condition ( Fig. 3A View FIGURE 3 ; Table 2 View TABLE 2 ). In the remaining two genera, either the third or second and third supraneurals are absent (the third is sometimes present as a vestige in some Pyronotanthias species , and all supraneurals are autapomorphically absent in N. carberryi ; Figs. 3B–D View FIGURE 3 ; Table 2 View TABLE 2 ). Reduction or loss of the third supraneural is not at odds with the proposed relationship of Nemanthias and Pyronotanthias to Anatolanthias (ADPF S/S/3/1+1), Luzonichthys (ADPF S/S/3/1+1 or S//3/1+1) and Rabaulichthys (ADPF S/S/3/1+1). However, although this character has been considered important in anthiadine systematics (e.g., Katayama 1959; Kendall 1976; Katayama & Masuda 1980; Anderson & Heemstra 2012), it appears to be homoplastic within the subfamily, occurring in all species of Anthias , Baldwinella , Choranthias , Hemanthias , Holanthias Günther, 1868 , Meganthias , Odontanthias , Pronotogrammus , Sacura and Tosana , and some species of Plectranthias , Pseudanthias and Tosanoides ( Pogonoski & Gill 2021) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Pyronotanthias
| Gill, Anthony C. 2022 |
Anatolanthias
| Anderson, Parin & Randall 1990 |
Anatolanthias
| Anderson, Parin & Randall 1990 |
Rabaulichthys
| Allen 1984 |
Rabaulichthys
| Allen 1984 |
Luzonichthys
| Herre 1936 |
Luzonichthys
| Herre 1936 |
A. mooreanus
| Herre 1935 |