Oedura jacovae, Couper, Patrick J., Keim, Lauren D. & Hoskin, Conrad J., 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.178483 |
|
publication LSID |
lsid:zoobank.org:pub:76A94039-C5D9-4008-A24C-16D317081973 |
|
DOI |
https://doi.org/10.5281/zenodo.5673976 |
|
persistent identifier |
https://treatment.plazi.org/id/1D566A09-FF8D-CF3B-FF47-FF1FE526BBB3 |
|
treatment provided by |
Plazi |
|
scientific name |
Oedura jacovae |
| status |
sp. nov. |
Oedura jacovae View in CoL sp. nov.
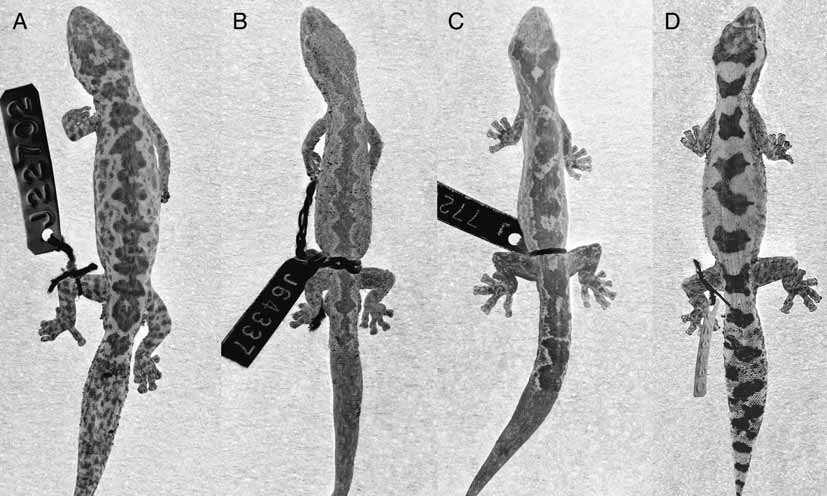
Figs. 1 View FIGURE 1 C, 2B, 3A and 4 Clouded Gecko
Material examined (all localities in SEQ): Holotype: QMJ77269 Mt Coot–tha, Brisbane (27°29’S, 152°57’E), L. D. Keim, March 2002. Paratypes: QMJ42169 Kroombit Tops (24°22’S, 150°59’E); QMJ66689 Kroombit Tops National Park (24°22’S, 151°01’E); QMJ50303-04 Kroombit Tops, Forestry Camp (24°26’S, 150°43’E); QM J63726 View Materials Kroombit Tops State Forest (24°28’S, 150°56’E); QMJ62063 Fraser Island, Lake McKenzie (25°27’S, 153°04’E); QMJ35873 Kauri Creek, via Bauple (25°49’S, 152°57’E); QM J50352 View Materials -53, QMJ50357-59 Toolara State Forest, eastern edge (25°57’S, 152°53’E); QMJ50450 Jimna State Forest, Marumbah Creek, Kundy’s Hut (26°42’S, 152°26’E); QMJ50354 Hell Hole Logging area, near Monsildale Mt (26°43’S, 152°20’E); QMJ30850 Cooyar (26°59’S, 151°50’E); QMJ62054 Mt Glorious, approx 200m down from PEI inct on Mt Nebo Rd. (27°22’S, 152°47’E); QMJ77430 Timbin Rd, Point Lookout, North Stradbroke Is (27°26’S, 153°32’E); QMJ22571, QMJ34591, QMJ36144-45, QMJ76304 Point Lookout, North Stradbroke Is (27°26’S, 153°32’E); QMJ5703 Brisbane (27°28’S, 153°01’E); QMJ22784, QMJ36147 Brisbane, Mt Coot–tha (27°29’S, 152°57’E); QMJ36143? Mt Coot–tha; QMJ24211 North Stradbroke Is., Dunwich (27°30’S, 153°24’E); QMJ2859 Redbank Plains (27°39’S, 152°50’E); QMJ77272 2.2km east of Southbrook township (27°41’S, 151°45’E).
Additional material — not types: QM J 50305 View Materials Mimosa Creek Camp, 2km north, Blackdown Tableland (23°48’S, 149°08’E); QMJ36146 Robinson Gorge, via Taroom (25°17’S, 149°09’E); QM J36135 View Materials -37 Leslie Dam, via Warwick (28°13’S, 151°55’E); QMJ31858 Inglewood, 33km west (28°34’S, 150°45’E); QMJ30733 Texas Caves, via Texas (28°53’S, 151°26’E).
Diagnosis: Oedura jacovae sp. nov. is a slender, medium-sized (max SVL = 62mm), well-patterned velvet gecko that occurs in SEQ and is most closely allied to O. lesueurii ( Fig. 1 View FIGURE 1 A) and O. rhombifer ( Fig. 1 View FIGURE 1 B). It is distinguished from its Queensland congeners by the following characters: 1st supralabial narrower than, or subequal to, the 2nd supralabial (Fig. 3A); 1st and 2nd supralabials equal in height or 2nd supralabial taller than 1st (Fig. 3A); generally with well-developed basal webbing between 3rd and 4th toe on hindlimb ( Fig. 2 View FIGURE 2 B); a dark, zigzag dorsolateral pattern (not strongly contrasting with base colour) encloses a broad, pale vertebral zone which is broken by 1–5 transverse lines between the fore and hindlimbs ( Figs 1 View FIGURE 1 C & 4); flanks, limbs and head without pale spots.
Etymology: jacovae ; for Jeanette Adelaide Covacevich, a former senior curator at the Queensland Museum, for her many contributions to Australian herpetology. The authors also recognise Jeanette as a prominent figure in Queensland conservation, particularly her efforts to preserve the unique character of North Stradbroke Island where O. jacovae sp. nov. occurs in open forest communities.
Description: SVL (mm): 26.6–61.6 (n = 30, mean = 48.8). Proportions as % SVL: T = 92.2–110.8 (n = 10, mean = 101.0); HL = 21.3–24.9 (n = 25, mean = 22.8); HW = 15.0–19.9 (n = 24, mean = 16.9); S = 9.1– 10.5 (n = 25, mean = 9.9); EE = 5.9–8.1 (n = 24, mean = 7.0); NL = 15.7–23.4 (n = 24, mean = 20.3); AG = 43.1–54.3 (n = 25, mean = 48.2); L1 = 26.9–33.8 (n = 25, mean = 29.9); L2 = 32.9–43.1 (n = 25, mean = 37.5). See Table 1 View TABLE 1 for summary of body proportions and scalation characters.
Head. Narrow, elongate, distinct from neck; head width 66.6–81.6 % head length (n = 29, mean = 74.1); head depth 40.0%–63.0% head width (n = 29, mean = 50.0); covered in small granules with slightly larger granules on the dorsal and lateral surfaces of the snout; 7–10 interorbital scales (n = 29, mode = 9); rostral approximately twice as wide as deep, undivided (3% of sample) partially divided (94%) or fully divided (3%) vertically by a medial groove; rostral shield contacting nostril, bordered by 2–4 scales along its dorsal edge (n = 30, mode = 3) and the 1st supralabial on each side; 5–6 scales bordering nasal opening (n = 30, mode = 6); supralabials 10–12 (n = 30, mode = 11), 1st supralabial narrower than 2nd supralabial (97% of sample, Fig. 3A), 1st and 2nd supralabials subequal in height (69 % of sample, Fig. 3A) or 2nd supralabial taller than 1st (31% of sample); infralabials 9–12 (n= 30, mode = 10) 4–7 rows of noticeably enlarged granules extending back from mental. Body. Slender, slightly depressed, covered in small granules; granules on ventral surface noticeably larger than those on dorsum; a row of 3–5 enlarged post-cloacal tubercules (n = 29, mode = 4) behind the lower posterior margin of the thigh in both sexes (better defined in males). Preanal pores present in mature males, extending to underside of thigh. Limbs. Moderate; digits dorsoventrally compressed and expanded distally each with an enlarged pair of apical lamellae followed by a transverse series, divided distally, single proximally; hindlimb with 5–8 enlarged lamellae (including apical pair) on 1st toe (n = 30, mode = 7), 5–9 on 2nd toe (n = 30, mode = 6), 6–8 on 3rd toe (n = 30, mode = 7), 5–8 on 4th toe (n = 30, mode = 7) and 5–7 on 5th toe (n = 30, mode = 6); basal webbing evident between digits of hindlimb, small–moderate between digits 2 and 3, a pronounced basal flange between digits 3 and 4. Original tail. Long and tapered ( Fig. 4 View FIGURE 4 A), slightly bulbous and carrot shaped in most specimens; scales arranged in concentric rings, slightly larger on ventral surface.
Pattern (in spirit) ( Fig. 1 View FIGURE 1 C): Colour pattern variable. Back and sides suffused with grey or chocolate brown. A dark-edged, zigzag stripe extends along dorsolateral zone from behind eyes to tip of tail; usually fragmented by dark transverse lines (1–5 between fore and hindlimbs, n = 23, mode = 4, mean = 3.3, SD = 0.97) that may produce a ladder-like pattern on back. Tail pattern similar to back, but tends to be more contrasting and more frequently broken by contact between the inner points of the zigzag edges. Head relatively plain or with dark marbling on top. A dark blotch often present on anterior neck that may form a short nuchal streak. Flanks usually with some indication of a dark mid-lateral stripe that is continuous with the facial stripe; beginning on snout, running through eye and above ear. Ventral surface pale; plain or marbled with brown along outer edge.
FIGURE 3. Arrangement of the first two supralabial scales in SEQ Oedura spp. Condition (A) O. jacovae sp. nov. (holotype, QMJ77269). The 1st supralabial is typically narrower (but may be subequal to) than the 2nd supralabial – a condition shared with O. robusta and O. lesueurii . In O. jacovae sp. nov. and O. robusta the first two supralabials are equally tall or the 2nd supralabial is slightly taller than the 1st. Condition (B) O. rhombifer QMJ64337. The 1st supralabial is usually wider (sometimes subequal to) than the 2nd supralabial. In O. rhombifer the 1st supralabial is taller than the 2nd supralabial. This is generally also the case for O. lesueurii , although in this species the 1st supralabial is only slightly taller than the 2nd.
Measurements and scale counts of holotype: QMJ77269 (female) SVL = 52.4 mm; T = (partially regenerated); HL = 11.9 mm; HW = 9.4 mm; HD = 4.2 mm; S = 5.2 mm; EE = 3.8 mm; NL = 12.2 mm; L1 = 14.1 mm; L2 = 19.3 mm; AG = 25.9 mm; Lamellae 1st toe 5, 2nd toe 6, 3rd toe 7, 4th toe 8, 5th toe 7; supralabials 12; infralabials 10; scales contacting dorsal edge of rostral 3; interorbitals 9; rows of enlarged postmental scales 6; scales bordering nostril 6; sales bordering posterior edge of mental 4; post-cloacal tubercules 4/3; rostral groove 0.5 of rostral depth.
Comparison with other taxa: In appearance O. jacovae sp. nov. can only be confused with three other species of Oedura in SEQ ( O. robusta , O. lesueurii , and O. rhombifer Figs. 1 View FIGURE 1 D, A and B respectively), all of which have a pale vertebral zone enclosed by a dark, wavy-edged dorsolateral zone/line running down either side of the back. In O. jacovae sp. nov. the dorsolateral zone tends to be less clearly defined and less regular than in the other three species ( Fig. 1 View FIGURE 1 C). A comparison of the vertebral pattern between O. jacovae sp. nov.
and these species follows:
• compared to O. robusta ; pattern not boldly contrasting and vertebral zone broken by several narrow cross bands ( Fig. 1 View FIGURE 1 C) vs. pattern boldly contrasting and vertebral zone broken by broad cross bands resulting in a series of pale blotches enclosed in a ladder-shaped longitudinal marking in O. robusta ( Fig. 1 View FIGURE 1 D).
• compared to O. lesueurii ; sides, limbs and snout without conspicuous spots ( Fig. 1 View FIGURE 1 C) vs. sides, limbs and snout usually with small spots and blotches in O. lesueurii ( Fig. 1 View FIGURE 1 A).
• compared to O. rhombifer ; vertebral zone broken by several narrow cross bands ( Fig. 1 View FIGURE 1 C) vs. vertebral zone usually clean in O. rhombifer ( Fig. 1 View FIGURE 1 B).
Differences in the relative widths and heights of the first two supralabials are also useful for diagnosing
these four species:
• In O. jacovae sp. nov. the 1st supralabial is typically narrower than, but may be subequal in width to, the 2nd supralabial (Fig. 3A). This condition is shared with O. robusta , O. lesueurii and some populations of O. rhombifer from coastal Queensland (Townsville–Hinchinbrook Island, Mackay area) and CYP, whereas in O. rhombifer (other than the aforementioned populations) the 1st supralabial is usually wider (sometimes subequal to) than the 2nd supralabial (Fig. 3B).
• In O. jacovae sp. nov. and O. robusta the first two supralabials are equally tall (Fig. 3A) or the 2nd supralabial is slightly taller than the 1st. This condition is shared with Townsville – Hinchinbrook Is., Mackay and CYP O. rhombifer populations. In O. rhombifer (other than the aforementioned populations) the 1st supralabial is taller than the 2nd supralabial (Fig. 3B). This is generally also the case for O. lesueurii , although in this species the 1st supralabial is only slightly taller than the 2nd.
Oedura jacovae sp. nov. usually has pronounced basal webbing between the 3rd and 4th toes ( Fig. 2 View FIGURE 2 B), as does O. robusta , but this is much less developed in O. lesueurii and largely absent in O. rhombifer ( Fig. 2 View FIGURE 2 A).
Oedura jacovae sp. nov. is of similar size to O. lesueurii , larger than O. rhombifer (max SVL 62mm vs. 52mm or 56mm if Alice Springs specimens included), and considerably smaller than O. robusta (maximum SVL = 62mm vs. 85mm). Oedura jacovae sp. nov. is further separated from O. rhombifer by the mean number of post cloacal tubercules (mean = 3.7, mode = 4, SD = 0.54, range 3–5, N = 29 vs. mean = 2.6, mode = 3, SD = 0.77, range 1–5, N = 227).
Genetics: A genetic analysis based on 400base pairs of mitochondrial ND4 mtDNA gene supports recognition of O. jacovae sp. nov. as a new species (Hoskin & Moritz, unpublished data). Sequences of O. jacovae sp. nov. from Mt Coot-tha (N = 2) and Kroombit Tops (N = 1) form a monophyletic group that is highly divergent (16–20%) from all other SEQ congeners: O. rhombifer ; O. lesueurii ; O. tryoni De Vis, 1884 ; O. monilis De Vis, 1888 ; and O. robusta . There is 3% divergence between the two Mt. Coot-tha (D’Aguilar Range) sequences (QMJ77269 and a tail tip from an uncollected individual) and the Kroombit Tops sequence (QMJ63726) of O. jacovae sp. nov. This level of divergence is relatively low compared to O. tryoni which displays approximately 11.5% divergence between the populations in the D’Aguilar Range and Kroombit Tops (Hoskin & Moritz, unpublished data).
Distribution: Oedura jacovae sp. nov. occurs in SEQ, with its core distribution between latitudes 24°– 28°30’ (Kroombit Tops–Warwick area, Fig. 5). In addition to the localities listed in the species account, there are also sight records from the following areas: Beerwah district ( Wilson and Knowles 1988, p. 240), and Moggill State Forest, Mt Crosby and Lockyer State Forest (CJH, pers. obs.). The greatest concentration of records lies within the greater Brisbane region, but this is more likely an artefact of high human population density in this area than a reflection of the local abundance of the species.
= geographically proximate populations of O. rhombifer
= geographically proximate populations O. lesueurii ? = specimens listed in`additional material’ (morphologically these conform most closely to O. jacovae sp. nov.) 1 = genetically sampled O. jacovae sp. nov. populations 2 = genetically sampled O. rhombifer populations
3 = genetically sampled O. lesueurii populations
= town
— — — = Queensland/NSW state border
We believe that the specimens listed in ‘additional material’ are also O. jacovae sp. nov., but have excluded them from the type series because of subtle differences in colour/pattern and the degree of webbing between the 3rd and 4th toes. These specimens come from the northern (Blackdown Tableland), western (Robinson Gorge) and south-western (Leslie Dam via Warwick, Inglewood area and Texas Caves) edges of the core distribution and individuals from these areas have not been sequenced.
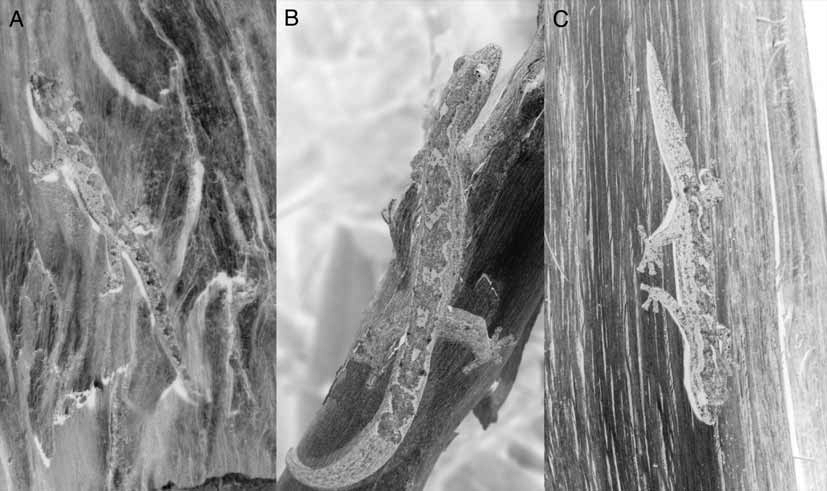
Habitat and habits: Oedura jacovae sp. nov. has been observed in dry open Eucalyptus forest, coastal woodlands and heaths, rocky outcrops, and urban areas bordering bushland. Figure 6 View FIGURE 6 shows a site where the species has been recorded, consisting of open Eucalyptus forest dominated by ironbarks ( Eucalyptus crebra ). The species appears to be predominately arboreal, being most commonly found at night foraging on vertical structures (e.g. tree trunks, rock faces, walls) and on the ground amongst leaf-litter or ground debris. In natural habitats O. jacovae sp. nov. shelters during the day beneath loose bark, in tree hollows, in rock cracks, or under ground debris. The species has been observed to co-occur in these habitats with the following gecko species: O. tryoni , O. robusta , Gehyra dubia ( Macleay 1877) , and the introduced Asian House Gecko Hemidactylus frenatus Duméril & Bibron, 1836 .
The occurrence of O. jacovae sp. nov. on human dwellings bordering native bushland communities is of interest. Surveys were conducted by one of us (LDK, 19/2–18/3 /2002) at Mt. Coot–tha Reserve (27° 29’S, 152° 57’E) as part of a study assessing the spatial distribution of the Asian House Gecko ( Hemidactylus frenatus ) across suburban/forest edges ( Keim 2002). During the course of these surveys, all sightings of O. jacovae sp. nov. were recorded and details of size and habitat use were taken. Twenty one observations of the species were made, although it is possible that some individuals were encountered more than once. Specimens ranged in size from 26–60mm SVL (N=12, mean = 54.6). Nineteen of the observations were made in association with houses, all of which backed directly onto the reserve. Here the geckos were observed at night on internal and external surfaces, clinging to wooden, concrete and brick walls, metal guttering, fly screens and window glass. They were recorded between 0.5 and 7 m above ground and from 1 cm to 2 m from sheltering sites. Only two specimens were observed in natural habitat during the course of these surveys, one beneath a fallen log during the day and the other on the trunk of a Spotted Gum ( Corymbia maculata ) at night. In this study, O. jacovae sp. nov. were not observed on houses that did not back directly onto bushland. Outside the survey period, O. jacovae sp. nov. was regularly seen in Mt Coot–tha Reserve on the trunks of C. maculata and E. crebra .
Conservation: Oedura jacovae sp. nov. is infrequently encountered, but appears widespread in SEQ. While common in some Brisbane streets adjoining Mt Coot–tha Reserve, O. jacovae sp. nov. does not appear to penetrate these suburban settings beyond the urban/bushland interface. Open eucalypt forests remain the core habitat for this species and these are rapidly being cleared in SEQ due to urban and industrial development. Such large-scale habitat removal will be greatly reducing and fragmenting populations of O. jacovae sp. nov., particularly in the Brisbane region.
The explosive expansion of the Asian House Gecko Hemidactylus frenatus through SEQ is also of concern. Originally native to Asia and the Indo-Pacific, this species was inadvertently introduced across much of the Pacific as well as parts of Africa and the Americas ( Case et al. 1994). Hemidactylus frenatus was first recorded in SEQ in 1983 (QMJ41978) and in less than twenty years has spread from Brisbane’s shipping wharves, through the greater metropolitan area, through the region’s urban centres, to homesteads and farm sheds in many rural communities. Hemidactylus frenatus is a highly successful competitor of other gecko species and has been implicated in declines of native geckos in Hawaii and Guam ( Petren et al. 1993; McCoid 1996) and is said to have displaced Gehyra australis Gray, 1845 and O. rhombifer from the house gecko niche in some areas of northern Australia ( Greer 1989, p. 66). Although Hemidactylus frenatus is abundant in urban environments in SEQ, it is currently rare in neighbouring bushland. However, Keim (2002) has shown that in the Darwin region of northern Australia, where H. frenatus has been present for a considerably longer period (arrived at Port Essington between 1838 and 1845, Greer 2006; first recorded in Darwin in 1964, Covacevich et al. 2001), it has successfully invaded native bushland communities. In this region, populations of H. frenatus were found in forest up to 250 m from the suburban edge ( Keim 2002). The impact of the species on native geckos was not quantified.
The impacts of H. frenatus on O. jacovae sp. nov. in SEQ remain unknown. While both species occur in sympatry in houses adjoining the Mt Coot–tha Reserve, this co-existence has only been for a relatively short time. Monitoring is required to assess the interaction between these two species and to assess whether H. frenatus can penetrate the forest communities that lie beyond the suburban edge and displace native gecko species, including O. jacovae sp. nov.
Future directions: This work arises from a larger study assessing phylogenetic relationships and phylogeography of eastern Australian Oedura spp. (Hoskin & Moritz, unpublished data). Further collections will enable the full geographic range of O. jacovae sp. nov. to be determined and will establish whether there are contact zones between O. jacovae sp. nov. / O. lesueurii and O. jacovae sp. nov. / O. rhombifer . Ongoing taxonomic work on O. rhombifer will focus on the Mackay coast, Townsville–Hinchinbrook Island, Cape York Peninsula, and Alice Springs populations (see Results).
TABLE 1. Body proportions (as % SVL, excluding juveniles) and scalation characters of O. jacovae sp. nov.
| Character Range | Mode | Mean | Standard deviation | N |
|---|---|---|---|---|
| SVL 26.6–61.6 | 48.8 | 8.70 | 30 | |
| Tail length % SVL 92.2–110.8 | 101.0 | 6.40 | 10 | |
| Head length % SVL 21.3–24.9 | 22.8 | 0.82 | 25 | |
| Head width % SVL 15.0–19.9 | 16.9 | 1.12 | 24 | |
| Snout % SVL 9.1–10.5 | 9.9 | 0.39 | 25 | |
| Eye-ear % SVL 5.9–8.1 | 7.0 | 0.51 | 24 | |
| Neck length % SVL 15.7–23.4 | 20.3 | 2.10 | 24 | |
| Axilla-groin % SVL 43.1–54.3 | 48.2 | 2.91 | 25 | |
| Forelimb % SVL 26.9–33.8 | 29.9 | 1.65 | 25 | |
| Hindlimb % SVL 32.9–43.1 | 37.5 | 1.81 | 25 | |
| Interorbital scales 7–10 | 9 | 8.7 | 0.92 | 29 |
| Scales bordering dorsal edge of rostral 2–4 | 3 | 2.8 | 0.46 | 30 |
| Scales bordering nasal Opening 5–6 | 6 | 5.9 | 0.25 | 30 |
| Supralabials 10–12 | 11 | 10.9 | 0.78 | 30 |
| Infralabials 9–12 | 10 | 10.5 | 0.86 | 30 |
| Post-cloacal tubercules 3–5 | 4 | 3.7 | 0.54 | 29 |
| Subdigital lamellae (hindlimb) 1st toe 5–8 2nd toe 5–9 3rd toe 6–8 4th toe 5–8 5th toe 5–7 | 7 6 7 7 6 | 6.5 6.8 7.1 6.6 6.1 | 0.86 0.94 0.48 0.76 0.68 | 30 30 30 30 30 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.