Branchinecta piurae, Alonso & Cohen & Ventura, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5162.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:9DCA74A7-48CF-4B32-AB3D-29DB12732278 |
|
DOI |
https://doi.org/10.5281/zenodo.6817945 |
|
persistent identifier |
https://treatment.plazi.org/id/1F0FA01F-0B58-FFC1-FF10-F8DCCD4BFB84 |
|
treatment provided by |
Plazi |
|
scientific name |
Branchinecta piurae |
| status |
sp. nov. |
Branchinecta piurae sp. nov.
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Etymology. The specific epithet “piurae” refers to ‘Department of Piura’ which is the Peruvian locality where the new species was found.
Type locality. Small pond located in Huancabamba Province; 6 km SE of Talaneo village; Department of Piura; Peru. 5°04’50.7”S, 79°29’26.4”W, altitude: 3,678 m a.s.l. GoogleMaps
Type material. Holotype. Undissected mature male preserved in 4% formaldehyde (Accession number: MNCN 20.04 About MNCN /11468), coll. M. Alonso, April 2019.
Allotype. Undissected mature female preserved in 4% formaldehyde (Accession number: MNCN 20.04 About MNCN /11469), coll. M. Alonso, April 2019.
Paratypes. 2 males and 2 females preserved in 4% formaldehyde (Accession number: MNCN 20.04 About MNCN /11470), coll. M. Alonso, April 2019 .
Comparative material examined. The new species was compared with fixed specimens in available collections and with the existing published descriptions of Branchinecta brushi , B. achalensis and B. papillata ( César, 1985; Cohen 1987, 2010, 2012; Rogers et al. 2008; Hegna & Lazo-Wasem 2010)
Diagnosis. Male. Second antennae long, extending to thoracic segments 5–7. Proximal antennomere with triangular spines, mainly in the distal half of anterior and medial surfaces. Distal antennomere, approximately two thirds length of proximal antennomere, slightly curved, with anterolateral and posteromedial edges denticulate, rounded at apex, with rasp-like surface. Basic structure of thoracopods as in genus. Genital segments with pair of well-developed ventral bulges hanging over basal non retractile part of gonopods. Basal non-retractile portion of gonopods with medial hook-shaped spine. Retractile part of gonopod typical for genus.
Female. Second antennae subcylindrical, ending in curved spine pointing anteriorly. Thoracic segments 1–10 provided with dorsal and dorsolateral protuberant grainy patches consisting in dense sets of tiny esclerotized scales. Brood pouch subcylindrical, rounded at end, not protruding laterally, extending to III–IV abdominal segment. Cysts spherical, with chorion faceted by polygonal fields.
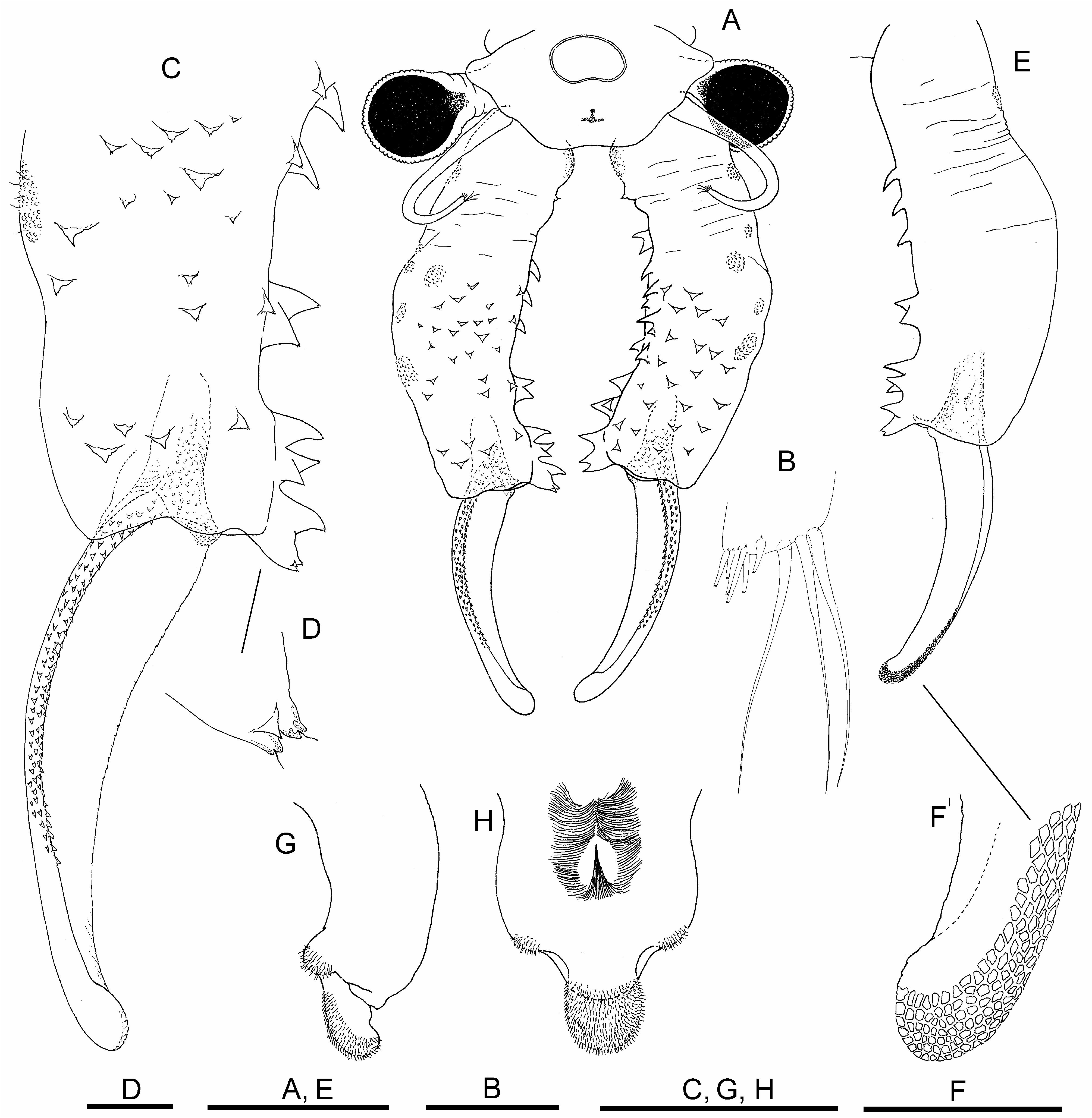
Description. Male. Body unpigmented. Head ( Fig. 1A View FIGURE 1 ) with lateral rounded expansions partly covering eyestalk. Nuchal organ well marked, elliptic with small concavity in its anterior margin. Compound eyes spherical, with diameter longer than corresponding eyestalk.
First antennae ( Figs. 1A, B View FIGURE 1 ) filiform, 2.3 times longer than compound eye diameter, about half shorter than proximal antennomere of second antenna; with three subdistal setae similar in length, about 2.5 times longer than first antenna width, with six distal short aesthetascs ( Fig. 1 B View FIGURE 1 ).
Second antennae ( Figs. 1A, C, E, F View FIGURE 1 ) extending backwards to thoracic segments 5–7. Spiny pattern of basal antennomere starting with group of anteromedial triangular spines pointing medially, continuing in distal direction of antennomere, with scattered similar shaped spines, pointing mediodistally or distally, over anterior and medial surfaces of antennomere. In addition, starting a little more proximal, row of stronger spines inserting along the medial posterior edge of basal antennomere; row initiating with a spine, sometimes bifid, followed in distal direction of antennomere by bigger conical, straight or curved spines; row ending in group of close spines, disorderedly mounted on distal posteromedial projection next to articulation with distal antennomere ( Figs. 1A, C, E View FIGURE 1 ). Tips of most spines ending in sensory hyaline seta ( Fig. 1D View FIGURE 1 ). Posterior surface without spines ( Fig. 1E View FIGURE 1 ). A2 distal antennomere ( Figs. 1A, C, E View FIGURE 1 ) approximately two-thirds length of proximal antennomere, concave in anteromedial surface, slightly curved, ending rounded at tip. Anterolateral edge of distal antennomere bordered by several parallel rows of small denticles, gradually flattening in distal direction of article, posteromedial edge barely denticulate, more evident in its proximal half. With rasp-like surface provided with esclerotized mesetiform cells, from distal part of posterolateral surface to rounded tip ( Fig. 1F View FIGURE 1 ).
Labrum ( Figs. 1G, H View FIGURE 1 ) subtrapezoidal, with posterior lateral setulose protuberances; lamella rounded, not very protruded, densely covered by setulae in distal-posterior surface. Dense setulose triangular pad placed midway on posterior surface.
Mandible asymmetric, as in most anostracans; posterior pole of both mandibles with tooth associated with one long thick and curved seta.
Maxilla 1, with 26 long setae, plus strong spine on limb ventral tip, as long as 3/4 length and 3 times width of basal part of long setae.
Maxillae 2, with 3 distal plumose setae, and 2–3 anteriorly directed densely plumose setae.
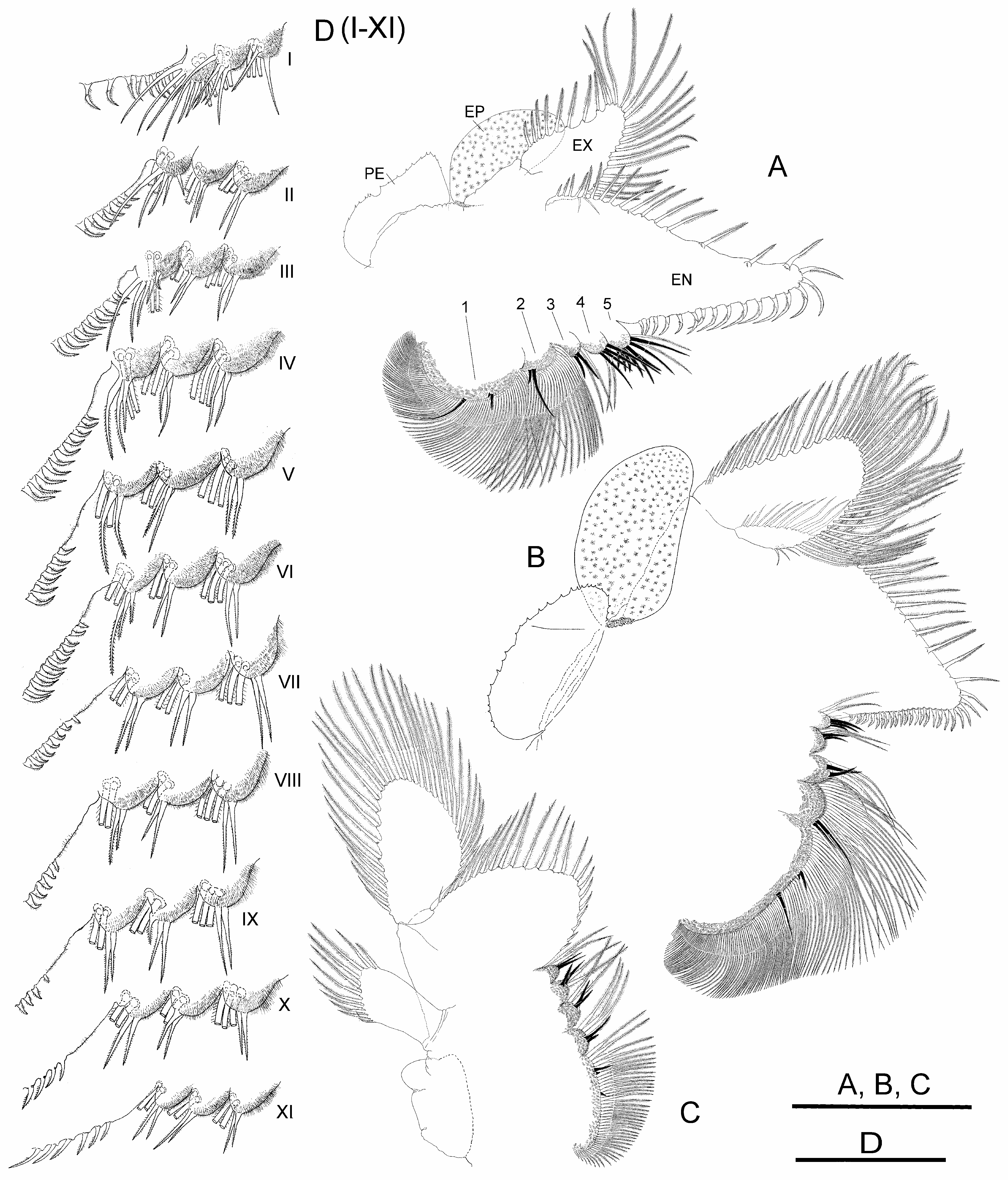
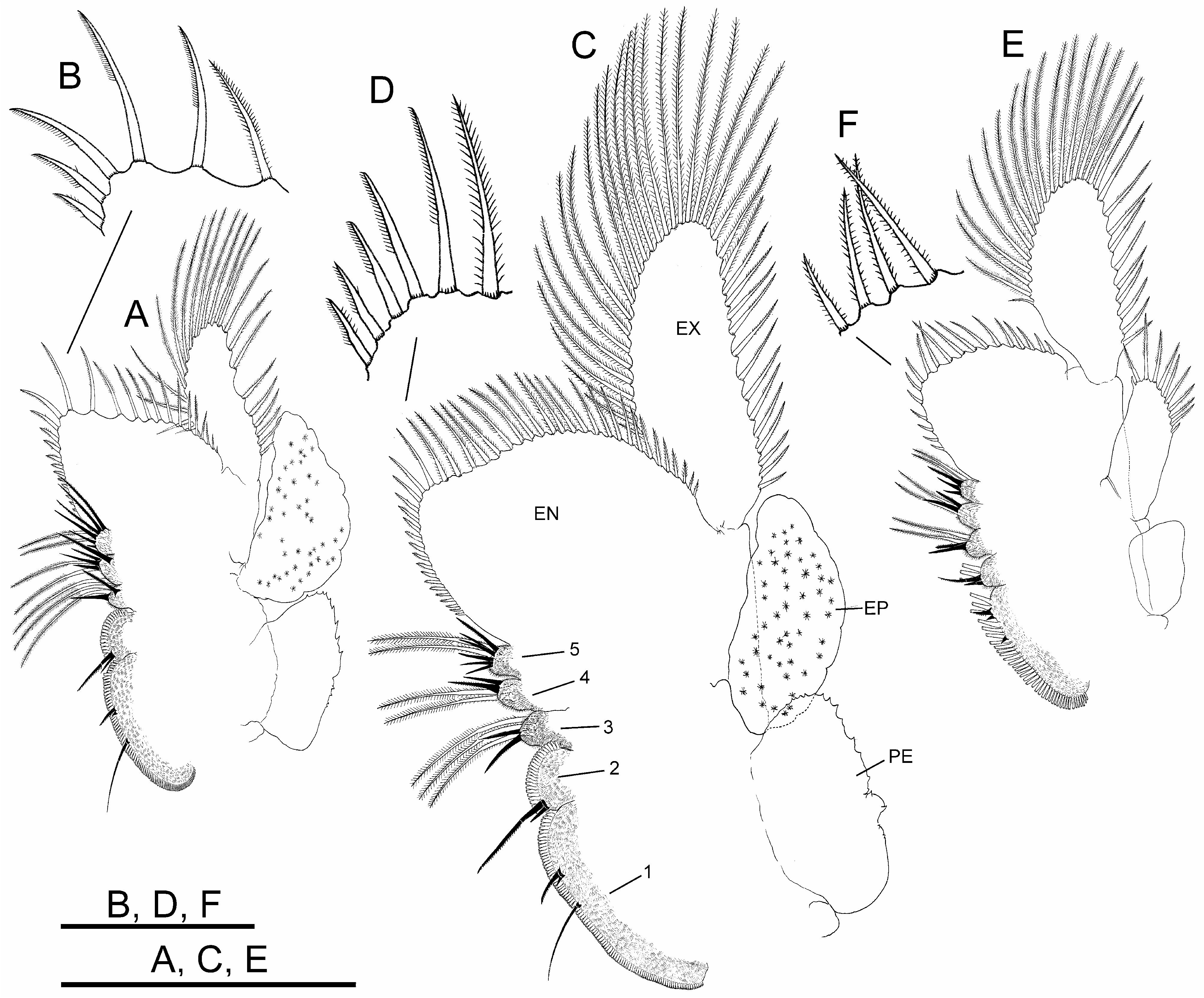
Thoracopods with gross structure typical for genus ( Figs. 2A–D View FIGURE 2 ). First thoracopod ( Fig. 2A View FIGURE 2 ) 0.8 times as large as fifth thoracopod ( Fig. 2B View FIGURE 2 ); eleventh thoracopod ( Fig. 2C View FIGURE 2 ) 0.7 times as large as first thoracopod.
Endopodite (EN) shape changing gradually throughout thoracopod series. First and second thoracopods ( Fig. 2A View FIGURE 2 ) with endopodite triangular, elongate, acuminate, 2.5 times length of exopodite. Third to ninth thoracopods ( Fig. 2B View FIGURE 2 ) with endopodite triangular, but less elongate, not acuminate, gradually shortening towards posterior thoracopods. Tenth and eleventh thoracopods ( Fig. 2C View FIGURE 2 ) with endopodite suboval and oval, respectively. First and second thoracopods ( Fig. 2A View FIGURE 2 ) with lower (distal) border with short and slender plumose setae, two most distal becoming stronger, slightly curved, bipectinate; apex and distal half of medial (inner) border with bipectinate strong setae very curved, bent towards proximal part of border; along proximal half of this border, setae becoming abruptly less curved and gradually decreasing in size (in length and width). Third to ninth thoracopods ( Fig. 2B View FIGURE 2 ) with setose armature similar to anterior thoracopods, but gradually weaker through thoracopod series; bipectinate setae along medial border gradually becoming less curved in direction to proximal part of border; thoracopods eighth to tenth backwards with most proximal setae on medial border curved in opposite direction; tenth to eleventh thoracopods ( Fig. 2C View FIGURE 2 ) with two most distal setae on lower border, and those at rounded apex of endopodite in tenth and eleventh thoracopods, weaker than in anterior thoracopods, bipectinate, straight.
Endites. Number and arrangement of anterior setae of endites 1 and 2 throughout thoracopod series ( Figs. 2A– D View FIGURE 2 ) as usual in anostracans ( Linder 1941). Number of anterior and posterior setae in endites 3, 4 and 5 of thoracopods shown in Table 2 View TABLE 2 ; number of anterior setae in these endites, proposed by Linder for Branchinecta is 2, 2, and 7–8 in the first thoracopod, and 2, 2, 3–7 in the rest, respectively, with acceptable deviation of ± 1 seta. First thoracopod with observed values agreeing with those of Linder in endites 3 and 5, but slightly higher in endite 4 ( Table 2 View TABLE 2 ). Intermediate thoracopods with observed values agreeing with those of Linder; 2–5 anterior setae observed on endite 5 in second to seventh thoracopods, decreasing to 2–3 in eighth thoracopods and subsequent ( Table 2 View TABLE 2 ).
Exopodite (EX) “D” shaped margined by plumose setae, slightly longer than or as long as epipodite in first to ninth thoracopods; up to seventh thoracopod, exopodite and epipodite of same width; eighth and ninth thoracopod exopodites becoming narrower than corresponding epipodites; tenth thoracopod with exopodite shorter and narrower than epipodite.
Epipodite (EP) of first to tenth thoracopods elliptical, with smooth margin; eleventh thoracopod, reduced, subrectangular, much shorter than corresponding exopodite, provided with 5–12 distal plumose setae in different individuals ( Fig. 2C View FIGURE 2 ).
Praepipodite (PE) of first to tenth thoracopods, with serrated edge and conspicuous marginal notch; eleventh thoracopod reduced to subtriangular lobe rounded at tip. Praepipodite shape changing gradually from first to fourth thoracopod, with angled distal margin ( Fig. 2 A View FIGURE 2 ), entirely oval from fifth to tenth thoracopod ( Fig. 2 B View FIGURE 2 ).
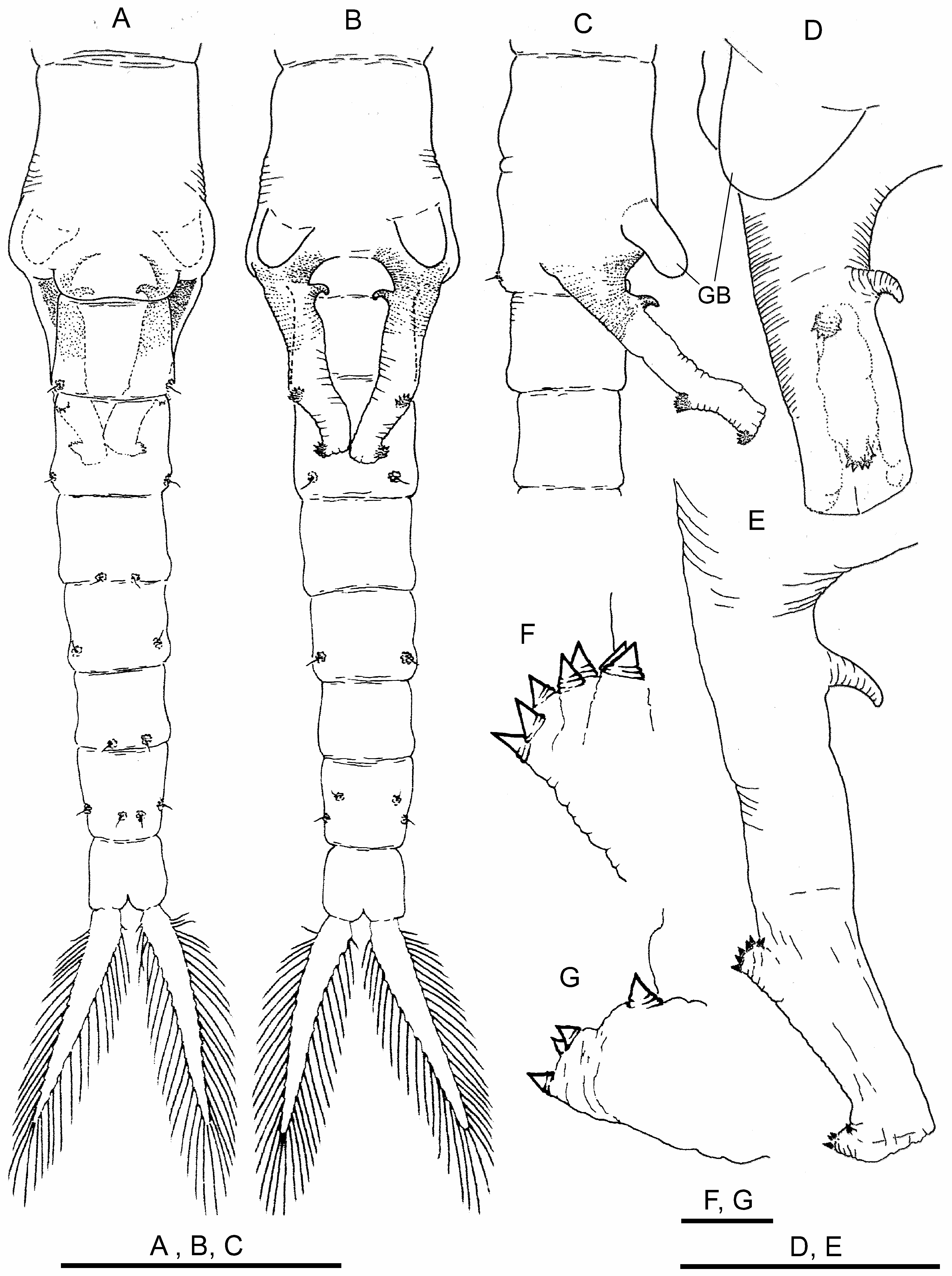
Genital segments ( Figs. 3A–D View FIGURE 3 ) provided with ventral pair of conspicuous bulges (GB) rounded at tip, easily seen in lateral view, hanging over basal part of gonopods. Basal non-eversible part of gonopods, with medial hooked spine ( Figs. 3B–E View FIGURE 3 ). Distal retractile part with two dentate warts as usual for genus ( Figs. 3E–G View FIGURE 3 ). Testis extending to III–IV abdominal segment.
Abdominal segments ( Figs. 3A, B View FIGURE 3 ) with thickened posterior border with pair of characteristic warty outgrowths, each with sensory seta.
Cercopods (uropods) seven times longer than broad at base and three times longer (setae excluded) than telson (anal segment), margined with plumose setae ( Figs. 3A, B View FIGURE 3 ).
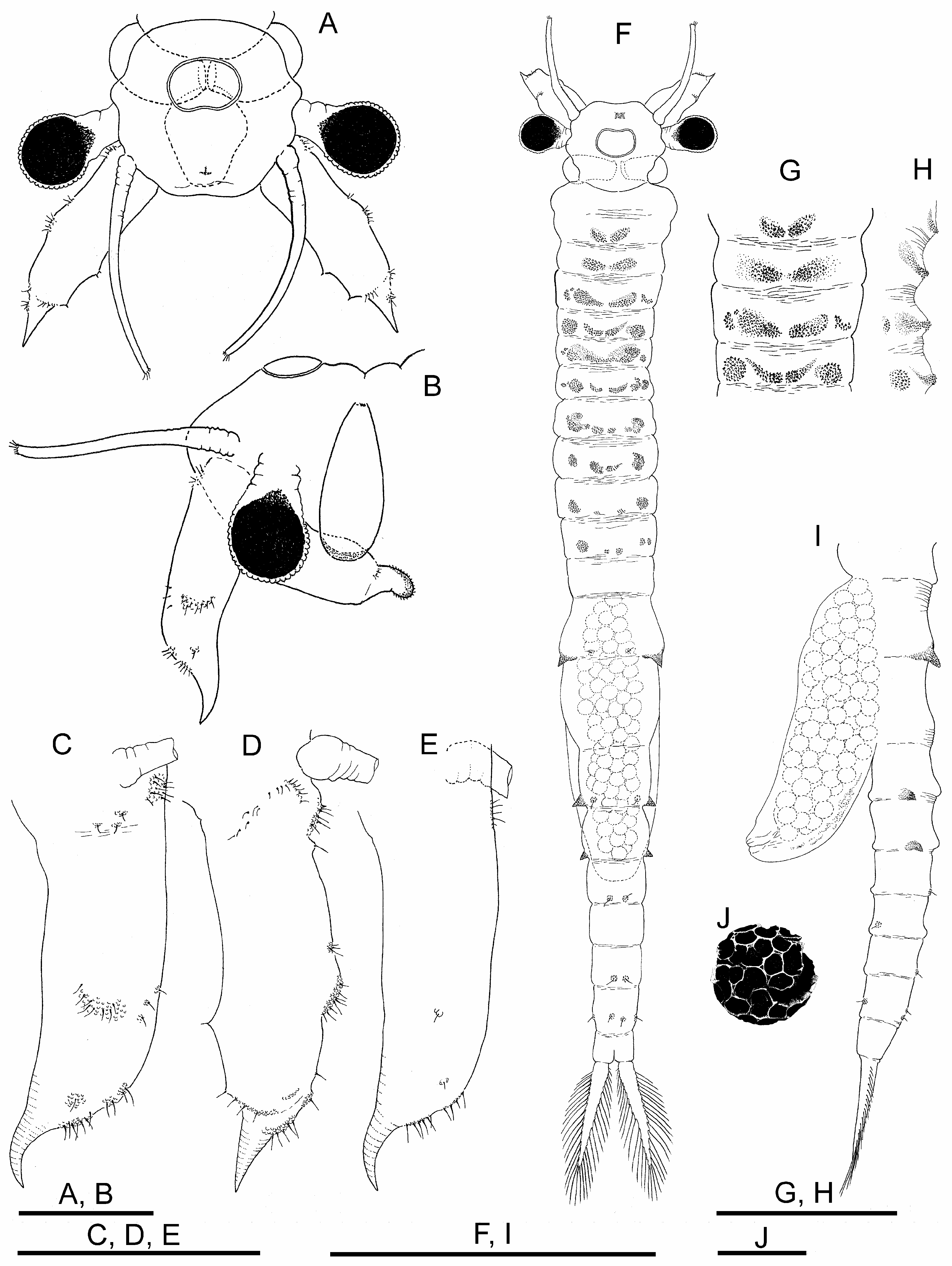
Female. Body unpigmented. Head ( Figs. 4A, B View FIGURE 4 ) without lateral expansions. Nuchal organ ( Figs. 4 A, F View FIGURE 4 ) as in male. Eyes with sexual dimorphism; although with diameter similar to male, peduncles shorter.
First antennae filiform, as long as second antennae excluding distal spine, and approximately three times as long as compound eye diameter ( Figs. 4A, B View FIGURE 4 ); subdistal setae and aesthetascs as in male.
Second antennae ( Figs. 4A–E View FIGURE 4 ) sub-cylindrical, ending in curved spine pointing anteriorly; with scattered usual verrucose sensory areas with hyaline setae; length (excluding distal spine) nearly three times width, spine fitting 4.5 times within appendage length.
Labrum as in male.
Mandible as in male.
Maxillae. Maxilla 1 as in male. Maxilla 2, with 6 distal plumose setae, and 2 anteriorly directed densely plumose setae.
Thoracic segments 1 and 2, with dorsal pair of low rough protuberances; thoracic segments 3 to 10, each with dorsal and dorsolateral pair of granular protuberances; dorsolateral protuberances reaching maximum size in segments 4 to 8 and decreasing in size until segment 10 ( Fig. 4F–H View FIGURE 4 ).
Thoracopods with sexual dimorphism in shape of endopodite and number and arrangement of its setose armature ( Figs. 5A, C, E View FIGURE 5 ).
Endopodite (EN) subrectangular in first to third thoracopods; suboval in fourth to ninth thoracopods; oval in tenth and eleventh thoracopods. First to tenth thoracopods with lower border with short, slender plumose setae; three–four most distal setae in apex becoming longer, with setulae only in one side, slightly curved ( Figs. 5B, D View FIGURE 5 ). First to third thoracopods with medial border bearing long bipectinate setae, slightly curved towards proximal part of endopodite, gradually decreasing in size in proximal direction, and more proximal setae curved in opposite direction; fourth to tenth thoracopods with all bipectinate and slightly curved setae bent towards apex of endopodite, gradually decreasing in size towards proximal part of border ( Fig. 5C View FIGURE 5 ). Eleventh thoracopod with setose armature along lower border similar to anterior pairs, but most distal long setae straight, with setulae on two sides; medial border with setation as in anterior pairs ( Figs. 5E, F View FIGURE 5 ).
Endites. Number and arrangement of anterior setae of endites 1 and 2 throughout thoracopod series ( Figs. 5A, C, E View FIGURE 5 ) as usual in anostracans ( Linder 1941). Number of anterior and posterior setae in endites 3–5 of thoracopods as shown in Table 2 View TABLE 2 ; number of anterior setae in these endites, proposed by Linder for Branchinecta is 2, 2, 7–8 on first thoracopod, 2, 2, 3–7 on second to eleventh thoracopods, respectively, with acceptable deviation of ±1 seta; first pair with observed values agreeing with those of Linder on endites 3 and 5, but slightly higher on endite 4, as in male. Intermediate thoracopods with observed values agreeing with those of Linder; eleventh thoracopod with number of anterior setae slightly lower, as in male.
Exopodite (EX), “D” shaped margined by plumose setae; in first to ninth thoracopods, slightly longer or as long as epipodite; in tenth and eleventh thoracopods, longer than epipodite.
Epipodite (EP) of first to tenth thoracopods oval, with smooth wavy margin; eleventh thoracopod, subrectangular, shorter and narrower than corresponding exopodite, with variable number (7–13) of distal plumose setae in different individuals.
Praepipodite (PE) of first to tenth thoracopods as in male; reduced to small triangular lobe rounded at tip on eleventh thoracopod.
Genital segments ( Figs. 4F, I View FIGURE 4 ). First genital segment with pair of strong lateral conical projections; second genital segment without projections. Brood pouch subcylindrical, not protruding laterally, rounded at tip, extending to III–IV abdominal segment ( Figs. 4F, I View FIGURE 4 ). Ovaries T-shaped, reaching posteriorly to abdominal segments I–IV in different specimens.
Abdominal segments. Abdominal segments I and II ( Figs. 4F, I View FIGURE 4 ), each with pair of rough lateral conical projections; those of second segment equal or somewhat smaller than those of first; both pairs of projections similar in shape but much smaller than those of first genital segment; in similar position, some specimens with additional pairs of rough, not pointed, much lower mounds on abdominal segments III and IV,.
Cysts. Diameter 220 μm; spherical; chorion faceted by polygonal fields ( Fig. 4J View FIGURE 4 ).
Cercopods (uropods) as in male.
Size. Total length of holotype (from the front to the end of cercopods without setae): 18.6 mm (head plus thorax 9.6 mm; abdomen 6.8 mm; cercopods 2.2 mm). Total length of allotype 17.8 mm.
Differential diagnosis. Branchinecta piurae sp. nov., B.papillata and B.brushi are the high altitude Branchinecta species recorded so far from South America. In addition, B. achalensis has to date been recorded from sea level to mid-elevation, not reaching altitudes beyond 2,200 m a.s.l. ( César 1985; Cohen 1987; Cohen 2012). Based on morphology, the closest species to B. piurae sp. nov. is B. papillata ( Cohen 2010, 2012). In both species, the male second antenna basal antennomere shows a posteromedial longitudinal row of spines starting approximately in the distal half of the antennomere and extending towards its distal joint ( Cohen 2012); gradually, the spines become bigger and curved pointing towards the extreme of the antennomere; the row ends in a distal posteromedial slightly swollen area which bears the strongest spines of the row ( Figs. 1A, C, E View FIGURE 1 ; Rogers et al. 2008: Fig. 3B View FIGURE 3 ; Cohen 2012: Figs. 1A, B View FIGURE 1 (PMS)). However, B. piurae lacks the proximal and distal anteromedial bulges with spines present in B. papillata ( Cohen 2010: Figs. 3a, b View FIGURE 3 (amp, amd); Cohen 2012: Figs.1A, B View FIGURE 1 (PAMB, DAMB)); instead in the distal half of its basal antennomere, males of B. piurae present scattered triangular spines over the anterior and medial surfaces ( Figs. 1A, C View FIGURE 1 ). However, the most striking similarity between the two species is the general morphology of the distal antennomere of the male second antenna, which is concave on the anteromedial surface, and ends rounded at the tip, although less curved in B. piurae than in B. papillata ( Figs. 1A, C, E, F View FIGURE 1 ; Rogers et al. 2008: Fig. 3 B View FIGURE 3 ; Cohen 2012: Fig. 1 View FIGURE 1 ). Both species share a peculiar character, such as the presence of denticles bordering both edges of this antennomere, very conspicuous in B. papillata , but only well developed in the anterolateral edge and barely in the posteromedial edge, in B. piurae ; denticles are shorter and wider in B. piurae than in B. papillata . In addition, both species present a distal rasp-like surface ( Figs. 1E, F View FIGURE 1 ). A small linguiform branching ( Cohen 2010: Fig. 3a, b View FIGURE 3 (rd); Cohen 2012: Fig. 1 View FIGURE 1 : SLB) at the base of the distal antennomere was not observed in B. piurae . However, it should be noted that the presence of that branching was overlooked in the original description of B. papillata ( Rogers et al. 2008) probably due to its position (see Cohen 2012). On the other hand, B. piurae lacks the lateral projections observed in B. papillata at the anterior edge of the head, between the bases of both second antennae, and projecting beyond the contour of the head ( Rogers et al. 2008: Fig 3B View FIGURE 3 ; Cohen 2012). The male genital segments ( Fig. 3 View FIGURE 3 ) are similar in both species, with a pair of conspicuous ventral bulges (GB) hanging over the basal part of gonopods ( Cohen 2012: Fig. 3A, B View FIGURE 3 ), and basal non-eversible part of gonopods bearing a similar medial hook-shaped spine.
In females of B. piurae , the dorsal surface of the thoracic segments are provided with well-marked rough protuberances mainly in segments 3 to 10, a characteristic that is not observed in B. papillata . In both species, the brood pouch is cylindrical or sub-cylindrical and rounded at the tip, but in B. papillata , it is longer and extends up to abdominal segments V–VII, while in B. piurae , it reaches abdominal segments III–IV ( Fig. 4I View FIGURE 4 ; Rogers et al. 2008: Fig. 2 C View FIGURE 2 ). Females of B. papillata show a pair of lateral globose projections (GP) on both genital segments, which are smaller on the second than on the first ( Cohen 2012), or are only present on the second genital segment (last thoracic segment, in Rogers et al. 2008); these lateral projections are much larger than those present on abdominal segments. On the abdomen, the lateral projections are observed from segments I to IV–V (gradually decreasing in size in caudal direction) (Rogers at al. 2008; Cohen 2010, 2012). Instead, B. piurae only presents a pair of strong lateral conical projections on the first genital segment. Similar projections, but smaller, are present on abdominal segments I and II ( Figs. 4 F, I View FIGURE 4 ), and in some specimens also on abdominal segments III and IV.
Branchinecta piurae differs from B. brushi in the general aspect of the second male antenna (Hegna & LazoWasem 2010). On the basal antennomere, B. piurae lacks the anteriomedial tubercle, and medial crest with blunt spines in the middle third of the antennomere observed in the last species. Furthermore, in B. piurae the distal antennomere, gouge-shaped and margined with denticles, is very different from that of B. brushi (Hegna & LazoWasem 2010; Rogers et al. 2020: Fig. 19.6 F). Branchinecta brushi differs from all other species of Branchinecta by its very short cercopods in both sexes, subequal in length to the last abdominal segment (telson) (Hegna & LazoWasem 2010).
Similarly to the medioposterior row of spines present in the male second antenna basal antennomere of B. piurae , B. achalensis shows a posterior row of strong curved spines on its medial surface from the middle of the antennomere to its distal joint ( Cohen 1987: Figs. 1d, e View FIGURE 1 ). However, both species differ completely in the remaining ornamentation of the basal antennomere ( Cohen 1987: Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ; 2012: Fig. 5 View FIGURE 5 ). In addition, the entire aspect of the distal antennomere is very different in both species. In particular, B. achalensis lacks denticulate edges and distal rasp-like surface ( Cohen 1987: Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ; Cohen 2012: Figs. 4B View FIGURE 4 , 5 View FIGURE 5 ). Female B. piurae lacks the conspicuous bulge with 1–5 minute spines or sensory setae present in the medial surface of the second antenna in B. achalensis ( Cohen 1987: Figs. 11, 16–21). The brood pouch in B. piurae is rounded at the tip, while in B. achalensis it is not blunt, and it very gradually becomes sharper toward the apex
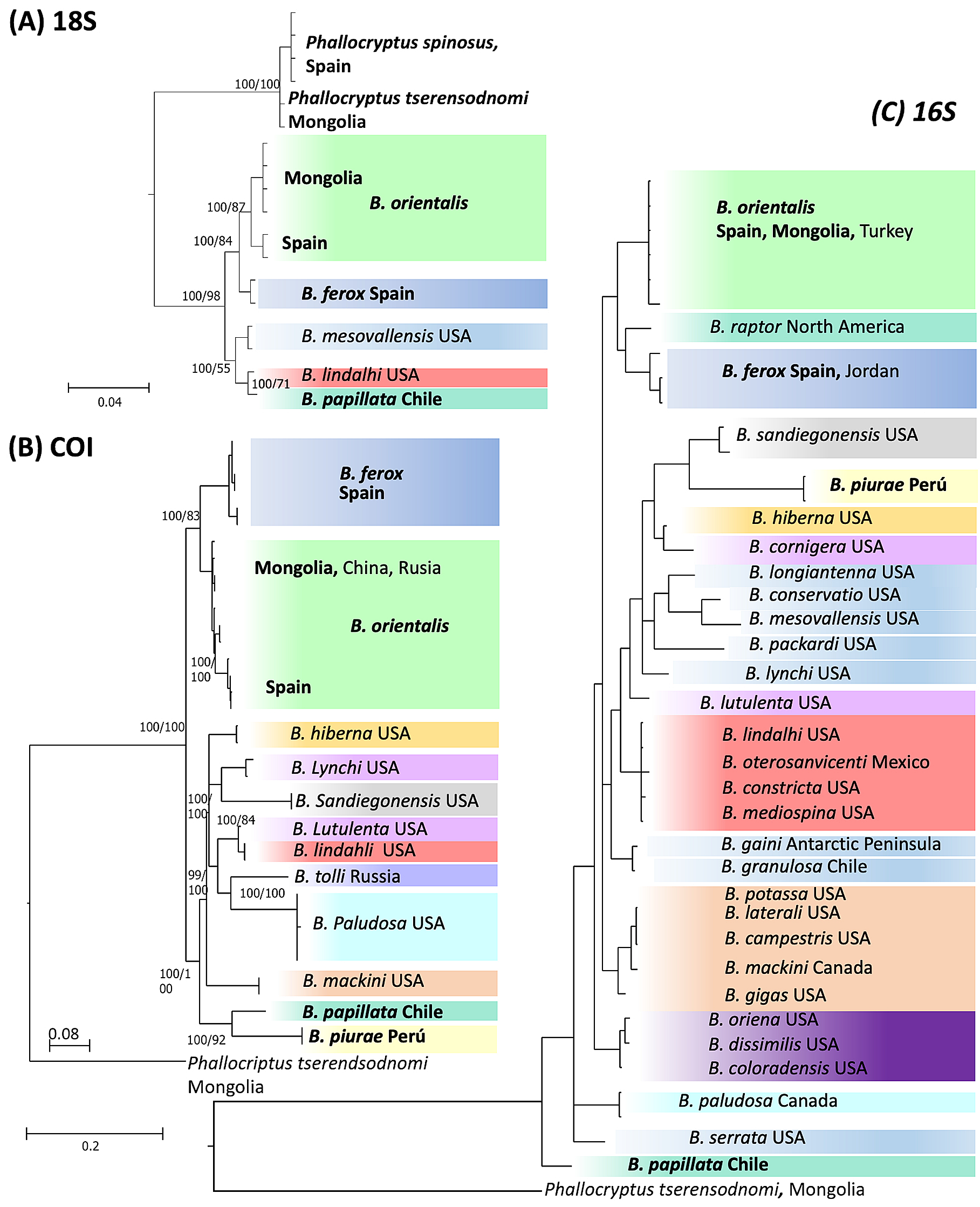
Genetic analyses. We were able to amplify DNA of Branchinecta piurae sp. nov. for COI and 16S together with B. papillata Rogers, De los Ríos & Zúñiga, 2008, also from South America; B. ferox (Milne-Edwards, 1840) from Europe; and B. orientalis G.O. Sars, 1901 from Europe and Mongolia ( Table 1 View TABLE 1 ). In contrast, B. piurae was the only species for which we were unable to sequence 18S. Overall, the three gene fragments generated different phylogenetic trees ( Fig. 6 View FIGURE 6 ) with B. piurae being clearly differentiated from the other species. The two Old World species Branchinecta orientalis and B. ferox were differentiated in the three trees, although being the sister clade to the American species only for 18S and COI. The mean genetic distance among the different species of Branchinecta was 16.3±4.9% (mean ± SD) for COI, 5.7±2.1% for 16S and 1.04±0.86 for 18S. The genetic distance between B. piurae and the other species of the genus was 19.3±2.7% and 10.3±2.1% for COI and 16S respectively, i.e. higher than the mean distances between species within the genus.
Distribution and ecology. As far as is known, B. piurae sp. nov. is an endemic species of the Peruvian and Ecuadorian Andes, appearing at altitudes around 3,700 –3,800 m.a.s.l, and latitudes from 2°47’ to 5°04’S. The Ecuadorian population was collected in the Cajas National Park in the frame of the project “Caracterización limnológica de los lagos y lagunas del Parque Nacional Cajas” sponsored by the Subgerencia de Gestión Ambiental de ETAPA and the Dirección de Investigación de la Universidad de Cuenca (DIUC) ( Alonso et al. 2017), and it could not have been recognized as a new species until the Peruvian population, which was collected afterwards and showed an identical morphology, could be taxonomically analysed in detail. The new species inhabits shallow temporary water bodies with rocky substratum and low or absent submerged macrophytes. Waters are transparent and very low mineralized (<100 µS cm-1 conductivity).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |