Nanotyrannus, Bakker et al., 1988
|
publication ID |
https://doi.org/10.3390/fossils2010001 |
|
DOI |
https://doi.org/10.5281/zenodo.10534261 |
|
persistent identifier |
https://treatment.plazi.org/id/201187CA-FFD8-FF80-FE21-FA7EFDA8FE74 |
|
treatment provided by |
Karina |
|
scientific name |
Nanotyrannus |
| status |
|
3.3. Nanotyrannus Morphology Inconsistent with Predicted Morphology of Juvenile Tyrannosaurinae
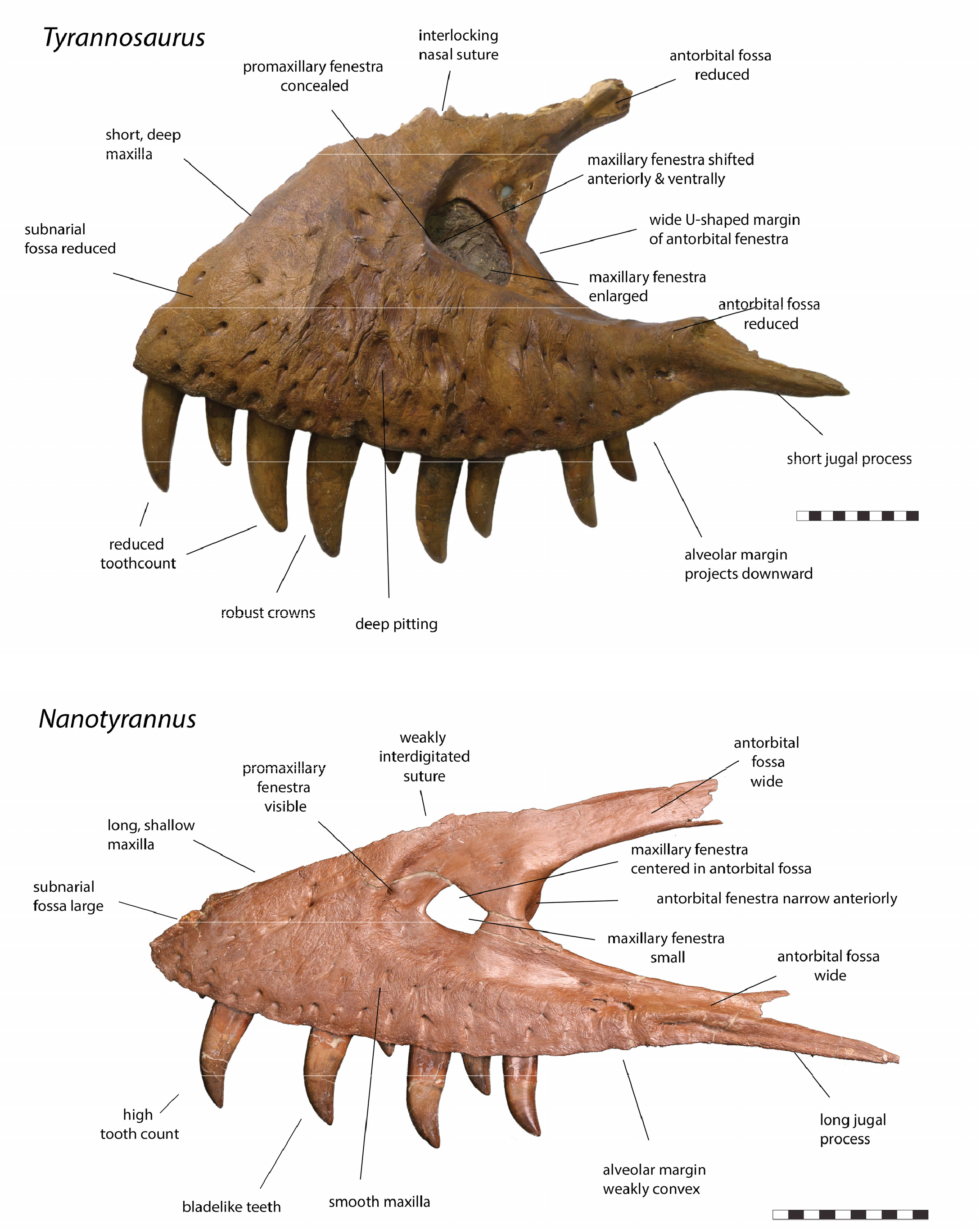
The hypothesized “growth series” linking Nanotyrannus to Tyrannosaurus can be tested by comparing it with the growth series of other tyrannosaurids, especially tyrannosaurines. If Nanotyrannus is a juvenile of Tyrannosaurus , and its distinctive morphology is the result of immaturity, then features of Nanotyrannus are predicted to occur in juveniles of other tyrannosaurs. If Nanotyrannus is a distinct taxon, then these features will be absent. We argue that juveniles of other tyrannosaurs do not conform to the “growth series” proposed for Tyrannosaurus [ 10].
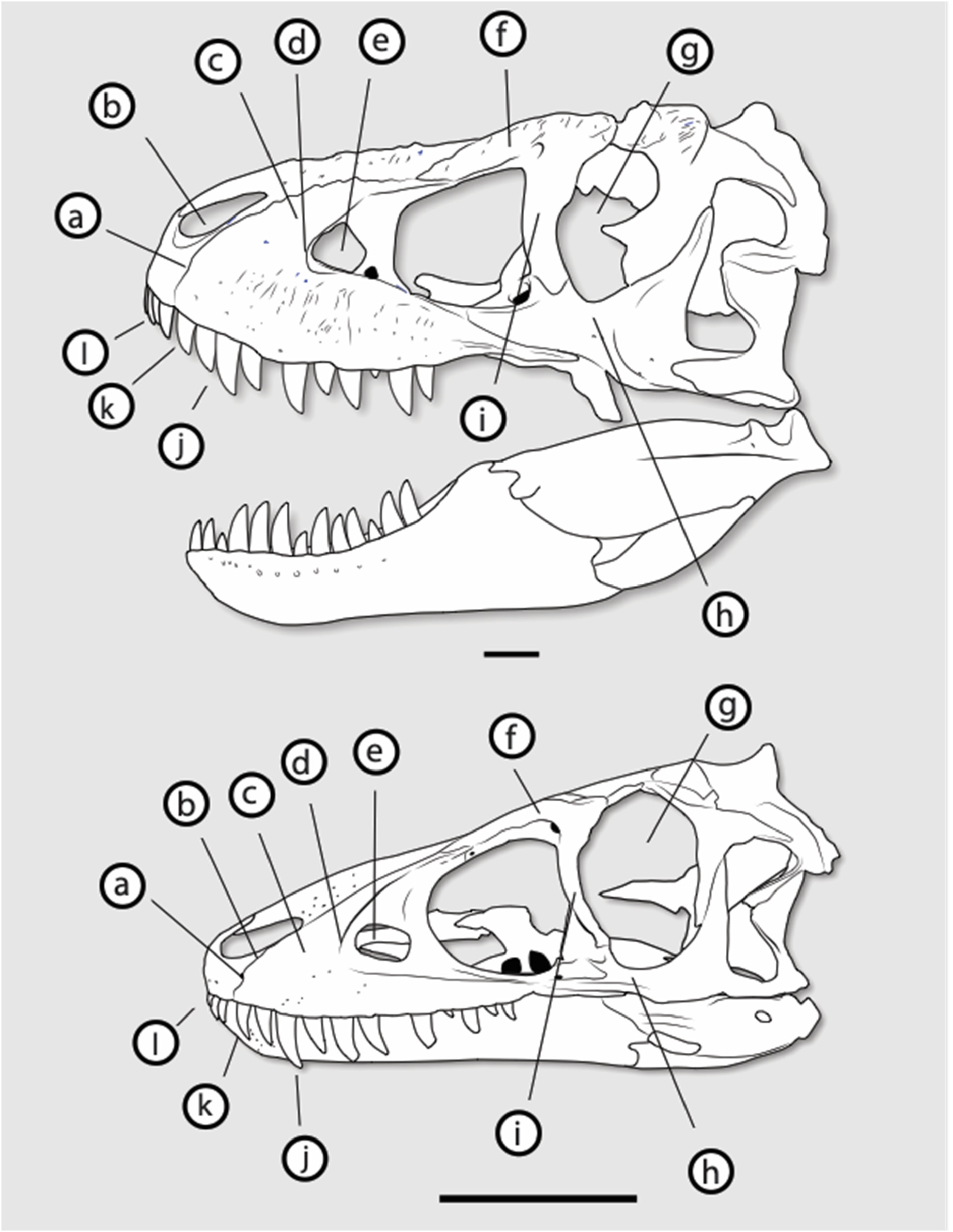
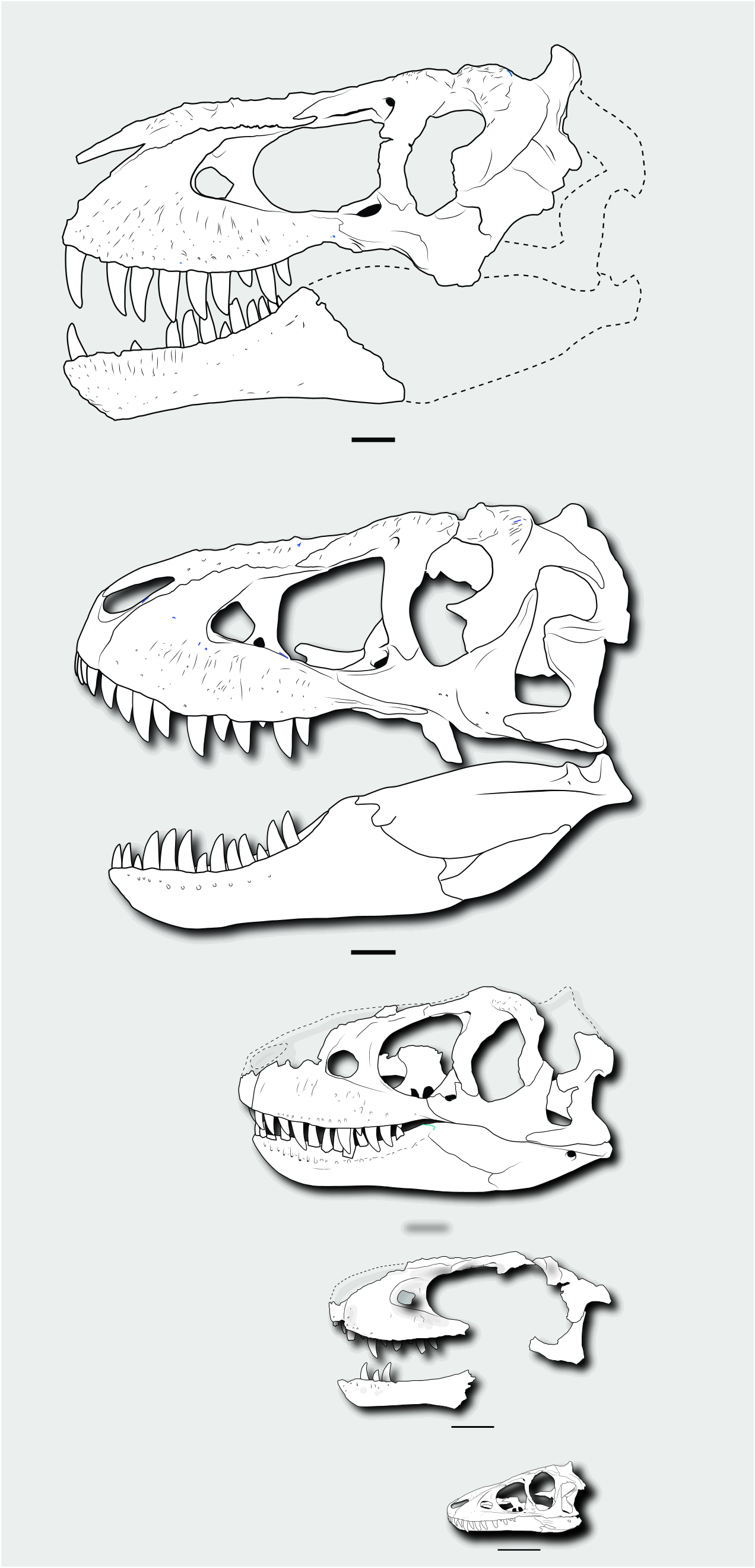
A young juvenile of Tarbosaurus bataar , a close relative of Tyrannosaurus , is known [ 54]. In several features—posteriorly wide nasals, a gracile postorbital, a slender dentary, and lack of the suborbital process of the orbit—the animal resembles Nanotyrannus . This means some features seen in Nanotyrannus could conceivably be juvenile characters, but these features do not necessarily mean that the animals are juvenile since they occur in adults of tyrannosauroids such as Alioramus [ 70, 96].
However, the juvenile Tarbosaurus skull differs from that of Nanotyrannus in many ways while resembling adult Tarbosaurus and T. rex ( Figure 19 View Figure 19 ). Features shared with Tarbosaurus and T. rex (but not Nanotyrannus ) include the tall and deep maxilla, the narrow rim of the antorbital fossa, an anteriorly placed maxillary fenestra, a large maxillary fenestra, limited contribution of the lacrimal to the antorbital fossa, weak curvature of the ventral ramus of the lacrimal, an anteriorly expanded jugal, and a broad base of the jugal postorbital process.
These features appear early in the ontogeny of Tarbosaurus and would presumably occur early in the ontogeny of Tyrannosaurus . The absence of these features in absolutely larger Nanotyrannus specimens is difficult to explain in terms of ontogeny unless Tyrannosaurus had a pattern of development unlike that of Tarbosaurus ( Figure 20 View Figure 20 ).
Some features of Tyrannosaurinae, especially those related to the skull ornamentation, orbits, and skull roof, appear to develop late, but others appear in even the youngest specimens ( Figures 19 View Figure 19 and 20 View Figure 20 ). At least some tyrannosaurine features would be expected in Nanotyrannus if it was a juvenile tyrannosaurine, but few, if any, are present.
Juveniles are also known for Gorgosaurus libratus , including skulls [ 109, 110] and isolated elements [ 9]. Juveniles are remarkably similar to adult Gorgosaurus , particularly in the shape of the maxilla, antorbital fenestra, antorbital fossa, and maxillary fenestra, implying that Gorgosaurus did not undergo radical changes in skull anatomy as it grew. Neither do juvenile Gorgosaurus exhibit Nanotyrannus -like characters such as the expanded antorbital fossa, procumbent premaxillary teeth, or a pneumatized quadratojugal. Growth patterns in Gorgosaurus , therefore, argue against Nanotyrannus ’ morphology being the result of immaturity.
For Nanotyrannus to be a juvenile Tyrannosaurus , Tyrannosaurus would have had to have a radically different development pattern than Tarbosaurus or Gorgosaurus . This is not impossible; ontogeny evolves. However, it is more parsimonious to treat Nanotyrannus and Tyrannosaurus as distinct species.
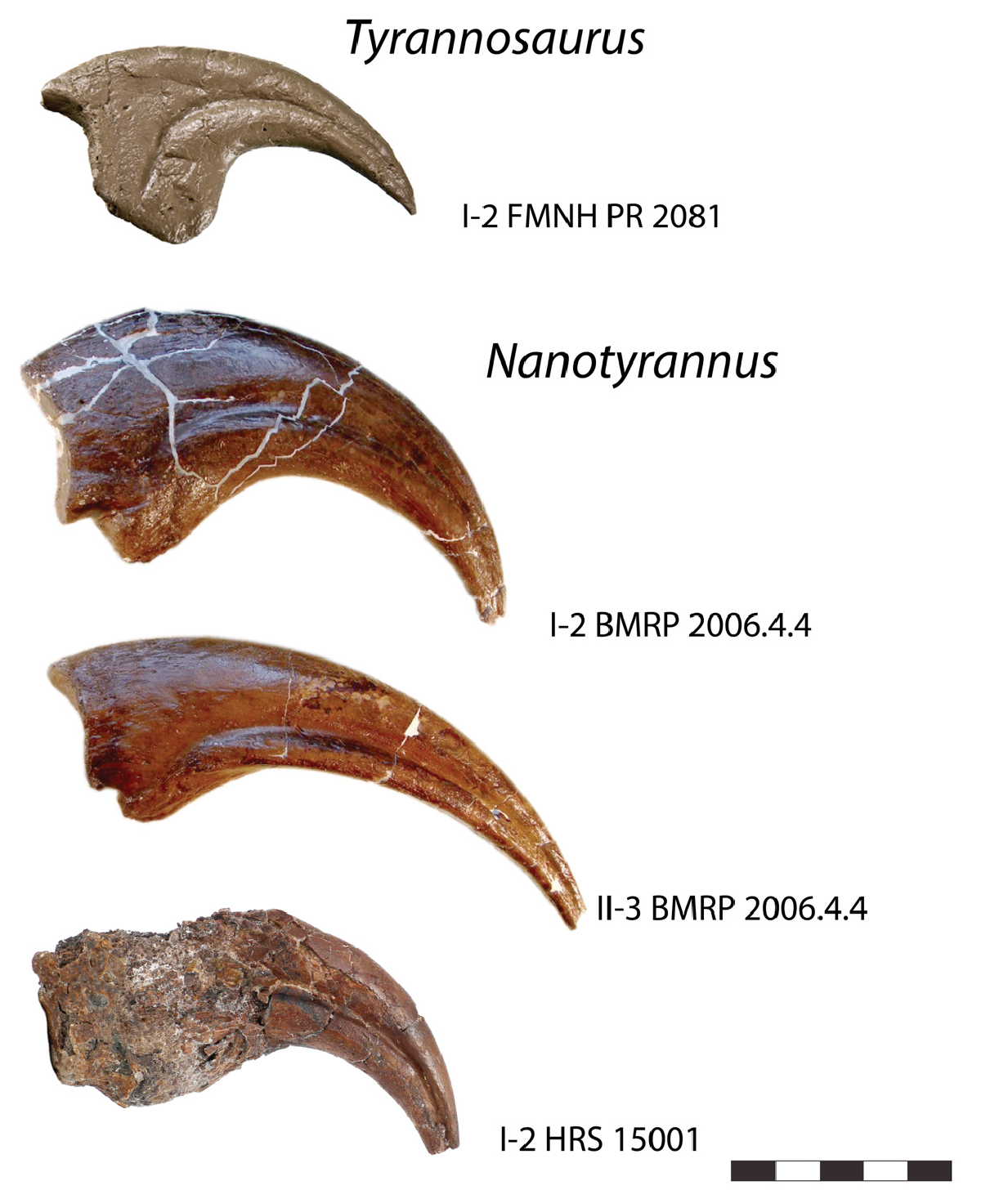
Finally, the proportions of the manus in the two animals are inconsistent with Nanotyrannus developing into Tyrannosaurus ( Figure 21 View Figure 21 ). Despite coming from much smaller animals, approximately 5–6 m in length (versus 12 m or more in Tyrannosaurus ), manual phalanges of BMRP 2006.4.4 and HRS 15001 are significantly larger than those of even very large Tyrannosaurus, FMNH PR 2081 . While allometric growth is possible, with the manus becoming proportionately smaller, the proportions seen in Nanotyrannus require the manus and claws to become absolutely smaller–for bone to be resorbed and elements reduced in length–as the animal matures. We are unaware of any amniote that develops in this fashion. Another problem is that the tip of the vomer is deeper in Nanotyrannus than in Tyrannosaurus ; this would require the end of the vomer to shrink or be resorbed [ 40].
Larson [ 40] also notes that patterns of pneumaticity are stable in birds as they grow, which makes differences in the presence and position of pneumatic foramina, such as the maxillary fenestra ( Figure 4 View Figure 4 ), difficult to explain.
3.4. Histology Supports Existence of Mature Nanotyrannus
3.4.1. Use of Histology to Test the Two Hypotheses
If Nanotyrannus is a juvenile of Tyrannosaurus , then all individuals showing the Nanotyrannus morphology must be immature relative to Tyrannosaurus . Bone histology can be used to infer the age and maturity of fossils, either to estimate absolute age (i.e., years of age) or relative maturity (e.g., young, rapidly growing juveniles and subadults, slower growing young adults versus old adults with slowed/ceased growth). The study of histology encompasses all aspects of bony tissue development, not simply thin sections and growth lines. To assess whether animals putatively identified as Nanotyrannus represent juveniles of T. rex or a distinct, small-bodied tyrannosaur taxon, maturity can be assessed in at least five distinct ways:
(i) Patterns of skeletal fusion;
(ii) Bone surface texture;
(iii) Presence/absence of an external fundamental system ( EFS);
(iv) Patterns of annual growth rates, either in terms of measures of bone deposition or kilograms of mass;
(v) Predicted adult mass, extrapolated from growth curves.
Nanotyrannus individuals show skeletal fusion and rugose facial bone, suggesting they were approaching maturity. Histology shows that Nanotyrannus individuals lack an external fundamental system, meaning that they are not old adults, but they show annual growth rates suggesting maturity. They also have predicted adult masses strongly suggestive of a distinct, small-bodied taxon rather than of juveniles of the giant Tyrannosaurus .
3.4.2. Skeletal Fusion
In vertebrates, composite elements such as the skull, vertebrae, shoulder girdle, sacrum, and pelvis may fuse late in development when growth slows. In crocodilians, centra and neural arches of vertebrae typically fuse late in life [ 111]. In ceratopsids, skull elements and their associated osteoderms fuse late in development [ 107]. Which elements fuse and the sequence of fusion can vary from taxon to taxon and even individual to individual [ 107]. Furthermore, some skull elements fuse early in ontogeny. The parietal bones, for example, are fused even in very young individuals in ceratopsids [ 112] and tyrannosaurids [ 54, 113], and nasals are fused even in very young tyrannosaurids [ 54, 113]. Therefore, not all fusions signal maturity. Some elements, however, only fuse in large individuals, suggesting their fusion correlates with skeletal maturity.
The scapula and coracoid fuse appear to fuse late in many dinosaurs, including Herrerasaurus [ 114], Abelisauridae [ 115], and at least some dromaeosaurs, including Velociraptor mongoliensis [ 116] and Achillobator giganticus [ 117]. Fusion of the scapulocoracoid also occurs in tyrannosaurs. Partial scapulocoracoid fusion is seen in Albertosaurus sarcophagus [ 71]; complete fusion is seen in a large T. rex [ 7] and a Tyrannosaurus from the Naashoibito member of the Kirtland Formation [ 118].
In some theropods, the pelvis shows partial or complete fusion in large individuals. The pubis and ilium fuse in the microraptorine Hesperonychus elizabethae [ 119]; the ilium, ischium, and pubis fuse in Coelophysoidea [ 120], Abelisauridae [ 121 – 123], and Ornithomimidae [ 124 – 127]. Fusion of the pubis and ischium also occurs in a large individual of T. rex [ 7]; the pubes and ischia are fused in Daspletosaurus UALVP 52981.
While not all skeletal fusions are correlated with maturity, fusion of the vertebrae, pectoral girdle, and pelvic girdle do seem to correlate with maturity. Strikingly, a number of fusions occur in Nanotyrannus BMRP 2002.4.1 [ 40]. These include fusion or partial fusion of neural arches to centra, fusion of the scapulocoracoid, and fusion of the ilium, pubis, and ischium [ 40]. This degree of skeletal fusion is consistent with the animal being a nearly full-sized subadult or early adult [ 40]. Further study of skeletal fusion patterns is needed for tyrannosaurs (and dinosaurs more generally), but evidence from skeletal fusions suggests that Nanotyrannus are not juveniles of Tyrannosaurus .
3.4.3. Surface Texture
In many dinosaurs, the adult skull bones take on a rugose to gnarled surface texture and may develop sculpturing. In chasmosaurine ceratopsians, for example, juveniles and subadults have smooth, striated skull bones. In adults, the bone takes on a gnarled texture, resembling tree bark, often with extensive, high-relief rugosity [ 107, 128, 129] and grooves for blood vessels. The appearance of rugose bone texture can be used as a rough proxy for maturity in Ceratopsidae . Striated bone is not seen in the very oldest individuals but is seen in very large individuals of Torosaurus [ 107], showing that it persists relatively late in subadults and young adults.
A similar pattern is seen in tyrannosaurids. In Gorgosaurus , nasals [ 9], maxillae [ 9], and postorbitals [ 110] are relatively smooth in juveniles, and become more rugose in subadults and adults. A similar pattern occurs in postorbitals of Daspletosaurus [ 110]. Young Tarbosaurus show weak sculpturing of the maxilla, while nasals and lacrimals are almost smooth [ 54]; smooth facial bones are seen in another juvenile tyrannosaurine, the holotype of “ Raptorex kriegsteini ” [ 113], likely a juvenile Tarbosaurus [ 55]. Adults have highly rugose facial bones [ 101].
These patterns are hard to quantify or characterize objectively, but overall, it appears that rugosity of facial elements increases as animals mature, providing a rough proxy for maturity. As in Ceratopsidae , striated bone persists relatively late in ontogeny, being seen in subadult Alioramus [ 70], young adult Gorgosaurus [ 109], and in the types of the tyrannosaurines Bistahieversor [ 59] and Thanatotheristes [ 110]. Although the presence of striated bone may show that an animal has not ceased growing entirely, its presence in relatively large young adults suggests that it cannot be used to identify animals as juveniles.
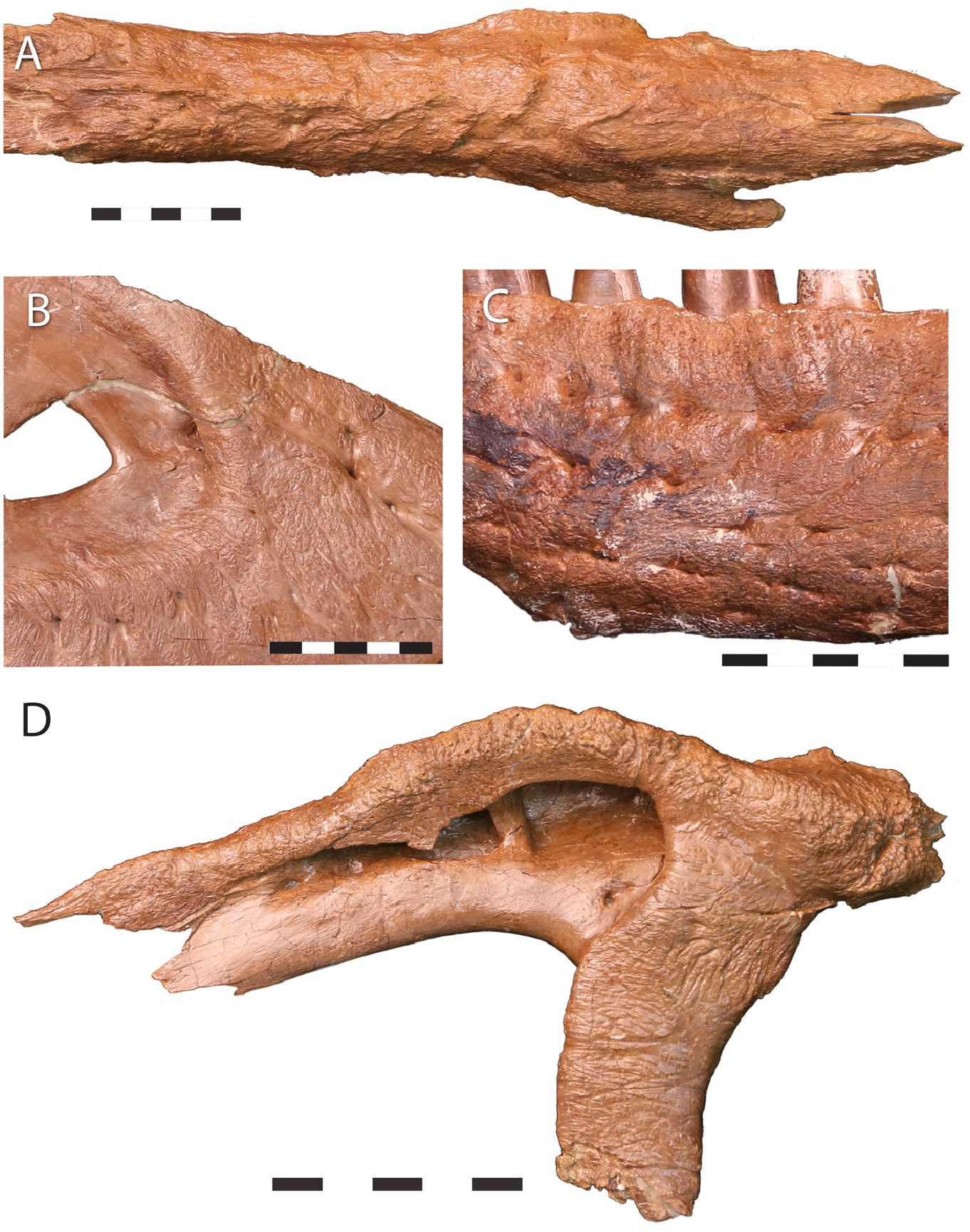
In the smallest Nanotyrannus specimen, LACM 28471, the surface of the maxillae and nasals is smooth, with little sculpture. However, in the larger N. lancensis holotype, CMNH 7541 ( Figure 22 View Figure 22 ), much of the nasals, maxillae, and the anteroventral surface of the dentary are rugose, as are the lateral surface of the lacrimal, the descending process of the postorbital, and the jugal ventral surface. Striated bone occurs inside the antorbital fossa, on the dentary’s dorsolateral surface, and the dorsal part of the jugal. The specimen, therefore, shows a mixture of textures, as expected for a subadult or young adult.
In Jane ( BMRP 2002.4.1), the maxillae, lacrimals, postorbitals, nasals, and the tip of the dentary are highly rugose and covered with grooves, sculpturing, and gnarled bone ( Figure 23 View Figure 23 ); striated bone is found on the antorbital fossa of the maxilla and lacrimal, the posterior end of the nasal, and the posterior end of the dentary. These bone textures suggest a subadult or young adult. The Zuri specimens show highly rugose sculpturing on the maxilla, nasals, lacrimals, and dentary tip. The maxilla of KU 155809 is also highly rugose. Meanwhile, the nasals of LACM 23845, the smallest definitive Tyrannosaurus skull, show weak sculpturing.
Striated surface textures associated with growth occur in the holotype of Nanotyrannus lancensis CMNH 7541 and in BMRP 2002.41. However, striated bone is seen in subadults or yong adults of other tyrannosaurs [ 57, 59, 70, 109]. Overall, bone textures suggest a degree of maturity in these animals, suggesting they are subadults or young adults of a distinct taxon, not juveniles of Tyrannosaurus .
3.4.4. External Fundamental System
The external fundamental system, or EFS, is an outermost band of very slow-growing bone with multiple, closely spaced lines of arrested growth (LAGs). It is deposited as growth rates slow and plateau late in life. An EFS can be used as an indicator of the cessation of significant growth and the attainment of maximum body size in a highly mature animal.
The existence of an EFS would be strong evidence that an animal was old and had effectively stopped growing. The absence of an external fundamental system would suggest that the animal had yet to achieve full adult size. It would mean the animal was not an old adult; however, given that the EFS appears late in life, as the animal attains maximum size [ 8, 49, 130], it would not preclude the possibility that an animal was a young adult just short of full size.
Three putative Nanotyrannus, BMRP 2002.4.1, BMRP 2006.4.4, and HRS 081514, have been sectioned and lack an EFS [ 46, 51]. This shows these animals are not old adults but does not preclude the possibility that these animals are young adults. In T. rex , individuals are nearly full size before establishing an external fundamental system. Sue, FMNH PR 2081, grew to an estimated 7930 kg before establishing an EFS [ 49, 50], then died at 8223 kg [ 50], adding only around 300 kg (i.e., <4% increase) after the appearance of the EFS. BMRP 2002.4.1, BMRP 2006.4.4, and HRS 081514 may represent young adults.
3.4.5. Growth Rates
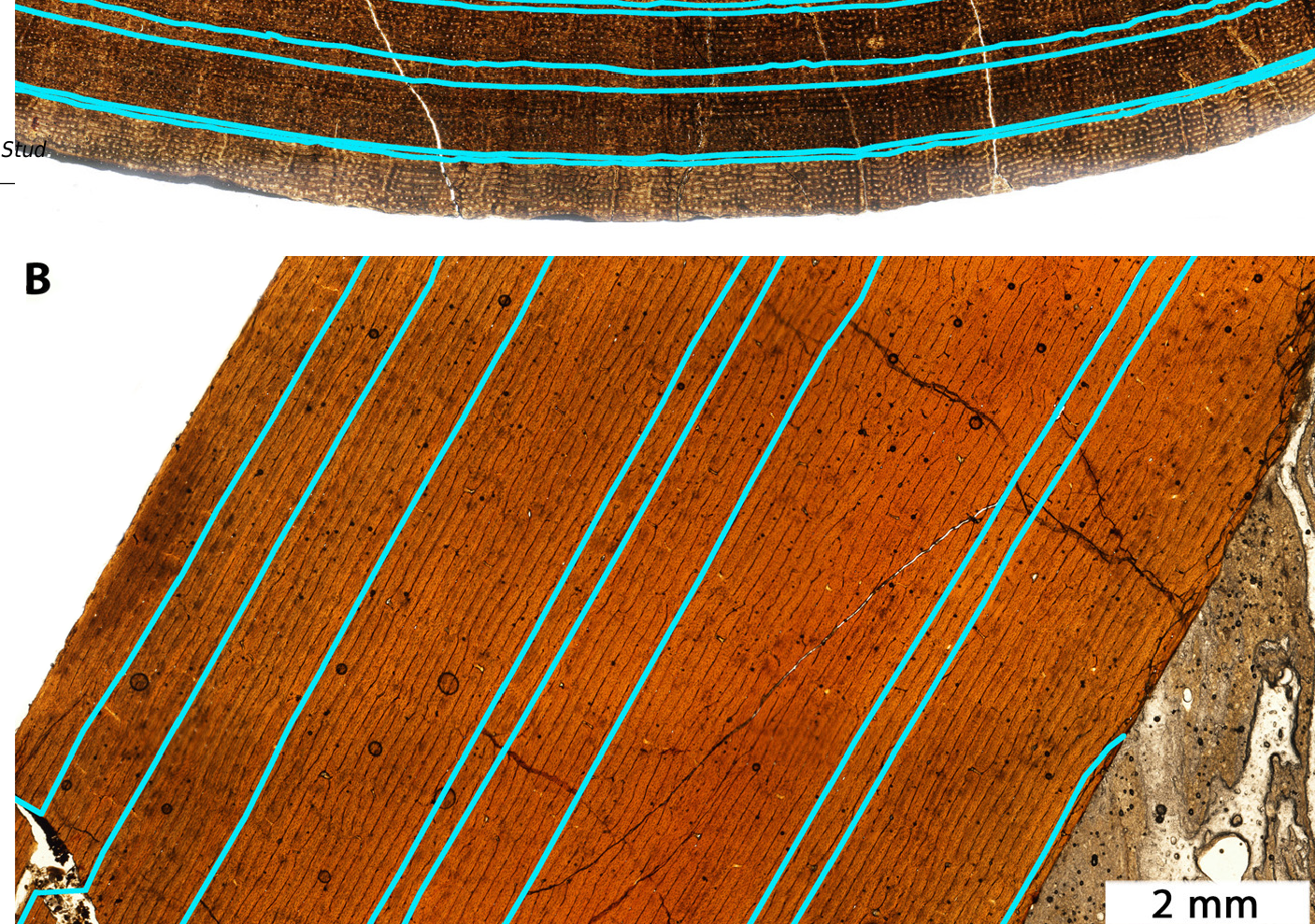
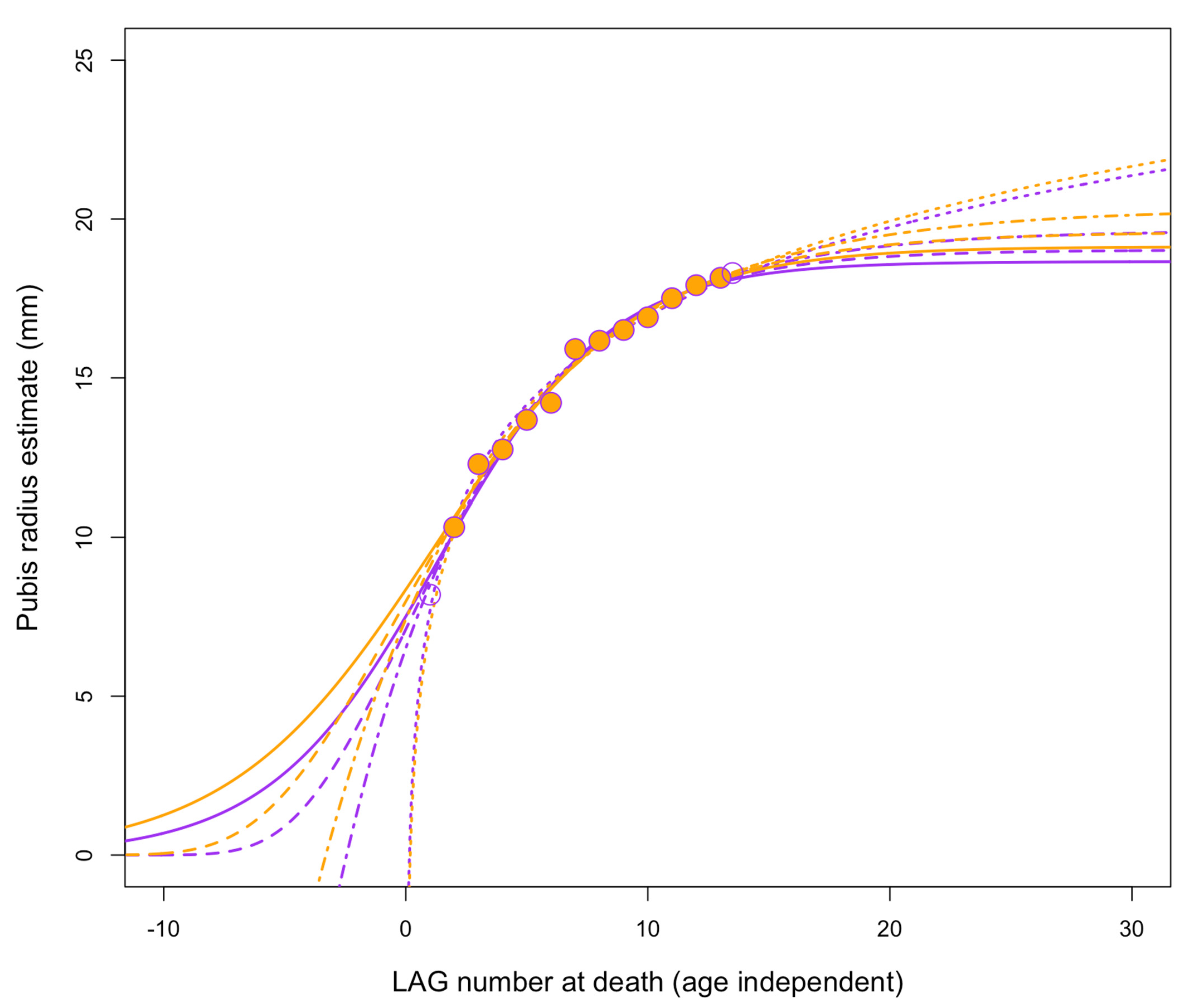
Lines of arrested growth (LAGs) record changes in bone circumference and diameter over time ( Figure 24 View Figure 24 ). Assuming that such lines develop annually, as is commonly done in paleohistology, it becomes possible to reconstruct growth rates by using measured and estimated circumferences either reported in or calculated from published data [ 46, 49, 50] to estimate mass [ 50] at various points in the individual’s lifespan. By converting femoral circumferences into body mass estimates [ 50], one can estimate changes in mass in terms of kilograms per year ( Table 5 View Table 5 ). Note that Jane’s femur is incomplete, so the circumference was approximated as a circle using LAG spacing from the endosteum and using femur width (Supplemental Material).
Juvenile tyrannosaurs have high maximal growth rates, approaching [ 8] or exceeding 800 kg /y in Tyrannosaurus . Immature Tyrannosaurus , particularly juveniles weighing ~ 1000–2000 kg, are predicted to have high growth rates as they enter their exponential growth phase. If Nanotyrannus is a distinct, small-bodied tyrannosaur, then it will have much lower growth rates at this size, comparable to growth curves modeled for small-bodied tyrannosaurids such as Gorgosaurus or Albertosaurus [ 8]. However, if the two taxa are synonymous, then specimens the size of BMRP 2002.4.1 and BMRP 2006.4.4 should be in their rapid, exponential growth phase—especially if one assumes that they have entered their teenage years [ 46].
Narrow spacing of LAGs ( Figure 24 View Figure 24 ), especially towards the periosteum, shows low growth rates in putative Nanotyrannus specimens ( Figure 25 View Figure 25 , Table 5 View Table 5 ). Growth rates do not exceed 150 kg /year for the last few years of life and can be less than 50 kg /year. This rejects the hypothesis that these are young, rapidly growing T. rex , which achieved peak growth rates exceeding 800 kg /y based on the estimates from FMNH PR2081 ( Table 5 View Table 5 ).
3.4.6. Growth Trends
Growth rates change over time. Growth accelerates until roughly the middle of life in symmetric logistic and asymmetric Gompertz growth models as commonly applied to dinosaurs [ 8, 131 – 133] then decelerates. Growth finally slows and almost stops in the last few years of life, reaching asymptotic growth. Based on their size (and even their approximated age from prior studies [ 46]), if the animals assigned to Nanotyrannus are juvenile T. rex , then they should show increasing growth rates (i.e., exponential growth) as they approach the rapid, protracted growth spurt at the middle of the tyrannosaur life cycle at around 1000–4000 kg [ 8]. If they are subadults or adults of a distinct, small-bodied species, they would be expected to show decelerating or ceased growth.
Growth rates in Nanotyrannus show a general trend of deceleration in their final years of life. These trends resemble those seen in mature T. rex as growth begins to plateau just before it establishes an EFS. These patterns are strongly suggestive of relatively mature animals, either late-stage subadults or early adults, not rapidly growing juveniles.
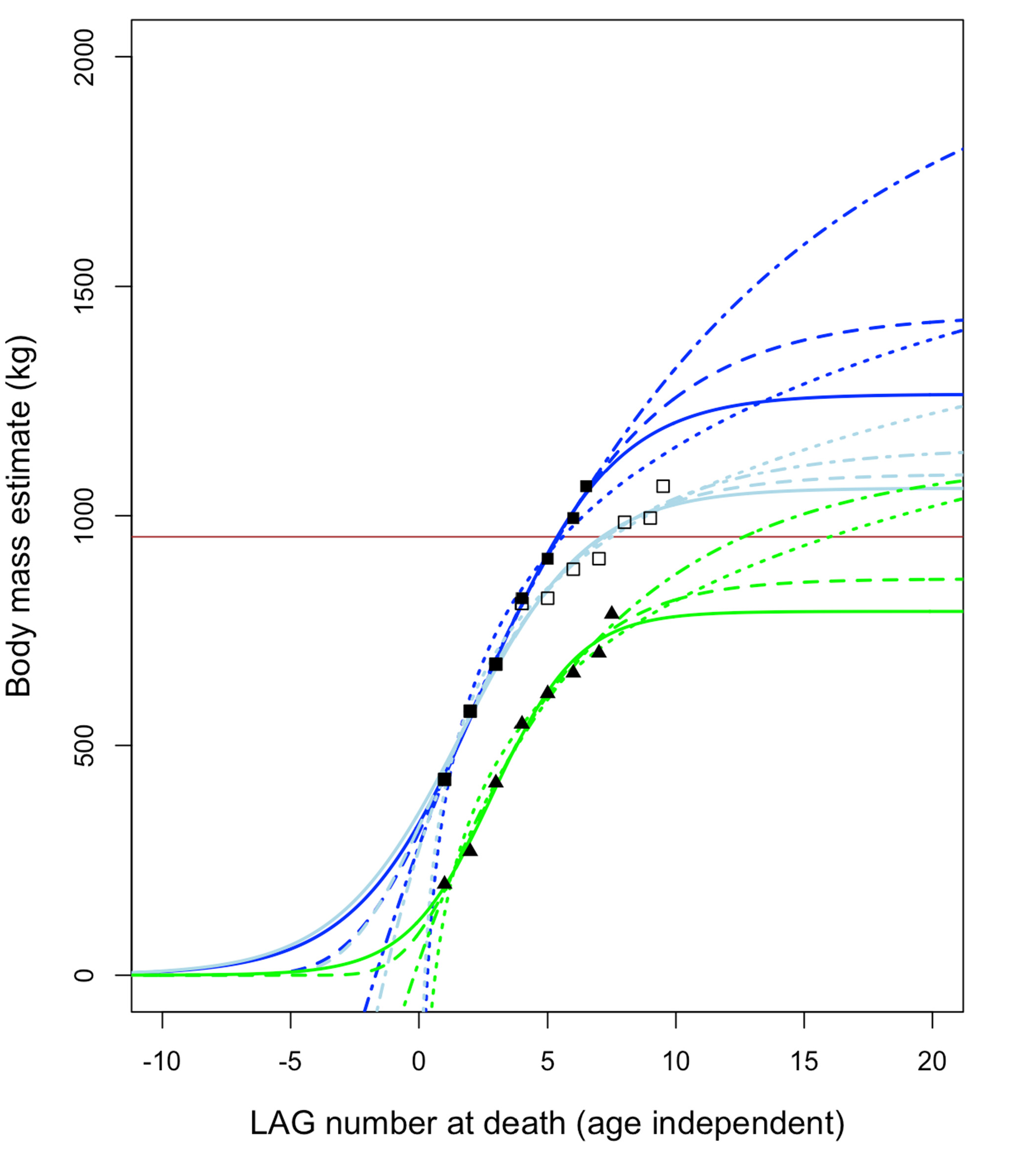
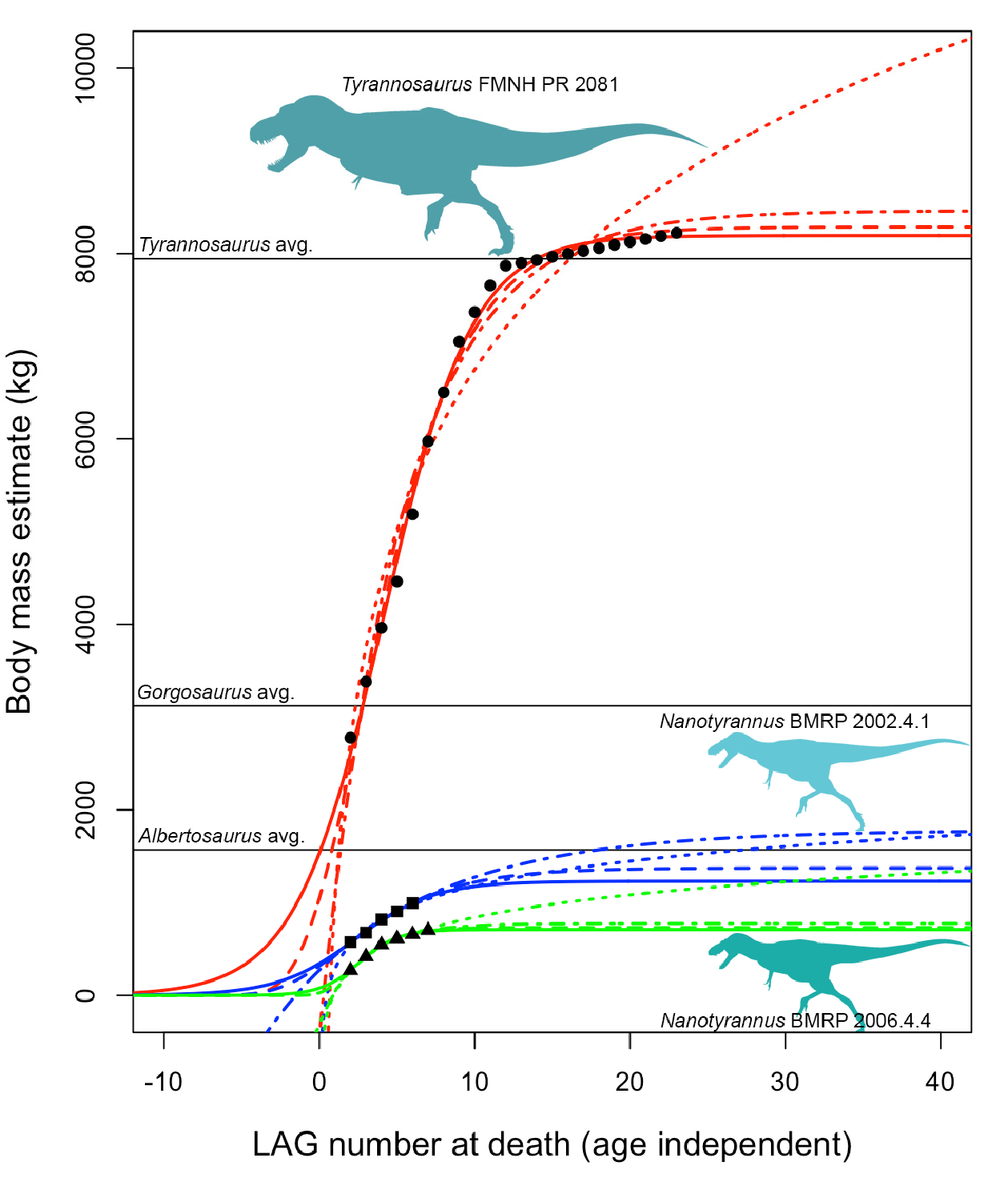
Estimated maximum size. It is possible to fit various kinds of growth curves to estimated masses [ 133], which can be extrapolated and used to predict the mass that a given individual would have achieved at full size. This approach can be used to test whether Nanotyrannus specimens would have grown to the enormous sizes (~ 8000 kg) seen in T. rex . If the putative Nanotyrannus were juveniles of T. rex , then their predicted adult masses should be on the order of 5000–10,000 kg, as in T. rex . If they are subadults or young adults of small-bodied tyrannosaurs, then their predicted adult masses should be much lower.
A caveat is that, when fitting a growth model to a year-by-year growth record from a single individual rather than to mass-age data from separate individuals, the assumption of independence of data is violated, making these pseudo-regression analyses (and making the calculation of confidence/prediction intervals moot). These models are nevertheless useful in extrapolating adult masses when individuals die prior to reaching full size. This is because, although extrapolation is always highly uncertain in science, we are limited to the growth record preserved in the femora; speculation that growth rates could have exponentially increased had these putative Nanotyrannus specimens lived longer is, therefore, a weaker argument than the use of adult size estimates from these pseudoregressions. We prefer the admittedly high uncertainty of extrapolation modeled on empirical evidence to speculation (i.e., one could speculate that any number of changes in growth rate or morphology might have occurred post-mortem since such speculation is unbounded by fossil evidence).
Growth curves using asymptotic logistic, Gompertz, and von Bertalanffy models predict fully adult masses ( Figure 26 View Figure 26 ) on the order of perhaps ~ 700–1100 kg for BMRP 2006.4.4 and ~ 1200–2100 kg for BMRP 2002.4.1 (when corrected for split multi-LAGs [ 49]). Non-asymptotic logarithmic models can achieve higher masses since they have no upper limit, but these predicted masses still fall far short of T. rex ( Figure 27 View Figure 27 ) and are closer to that of Albertosaurus . These estimates are, in the context of comparison with T. rex , also roughly consistent with mass estimates at the time of death for BMRP 2002.4.1 derived from 3D modeling [ 104]. Mass estimates are not available for the Zuri specimen ( HRS 081514) because the pubis was sectioned rather than the femur. However, plotting the growth of this specimen using data from Griffin [ 51] shows slow growth and growth deceleration rather than rapid, accelerating growth ( Figure 28 View Figure 28 ); it was apparently near full size when it died.
All mass estimates for adult Nanotyrannus are far below those expected for T. rex ( Figure 27 View Figure 27 ), which is predicted to hit ~ 8000 kg or more depending upon the model and mass estimates used. Growth trajectories of BMRP 2002.4.1 and BMRP 2006.4.4 are, therefore, inconsistent with their identification as juvenile Tyrannosaurus rex , even under a variety of growth models and initial conditions during curve fitting (Supplemental Material). Our estimates instead suggest that they represent a distinct, small-bodied taxon. Although it is conceivable that young Tyrannosaurus sometimes showed slow growth rates due to sickness, lack of food, or other stresses, it is unlikely that all three individuals sectioned would exhibit similar growth anomalies; it is more likely that they exhibit typical growth rates for their taxon.
Another alternative hypothesis for this variation in growth trajectories, while assuming taxonomic synonymy, would be that the putative Nanotyrannus specimens are members of the smaller sex in T. rex . While it is reasonable to assume that Sue is fairly representative of average adult T. rex size for its sex (i.e., as far as fossil discovery approximates random sampling of the population of T. rex ), the magnitude of hypothetical body mass dimorphism between Sue and the Nanotyrannus specimens from at least the asymptotic models (Supplemental Material) would be implausible. This hypothetical dimorphism would exceed those estimated or observed in other non-avian [ 134] and avian [ 93] dinosaurs, highly sexually dimorphic mammals such as sperm whales [ 135], and would only be comparable to the most extreme examples of sexual dimorphism in extant tetrapods (e.g., southern elephant seals [ 136, 137]).
| BMRP |
BMRP |
| HRS |
HRS |
| FMNH |
USA, Illinois, Chicago, Field Museum of Natural History (also used by Finnish Museum of Natural History) |
| UALVP |
UALVP |
| LACM |
USA, California, Los Angeles, Los Angeles County Museum of Natural History |
| CMNH |
USA, Pennsylvania, Pittsburgh, Carnegie Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.