Ercolania kencolesi, Grzymbowski & Stemmer & Wägele, 2007
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1577.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:8F6D8CD8-EE81-484F-922F-CD1E58E3A0AE |
|
persistent identifier |
https://treatment.plazi.org/id/225C3555-7548-BD46-FF64-FCB6FC24F867 |
|
treatment provided by |
Felipe |
|
scientific name |
Ercolania kencolesi |
| status |
sp. nov. |
Ercolania kencolesi View in CoL sp. nov.
Repository
Australian Museum , Sydney : Type material: Casuarina Beach, Lizard Island, North Queensland, Australia; shallow water up to 1 m. 2 nd July 2006. Holotype C.209735, length of preserved specimen 2.3 mm; 1 paratype C.209736, length of preserved specimen 2.1 mm;
Further material: Casuarina Beach, Lizard Island , North Queensland, Australia; shallow water up to 1 m depth. 30 th June 2006 (1 specimen used for histology), 2 nd July 2006 (1 specimen used for molecular studies), 7 th June 2006 (1 specimen used for molecular studies) .
Localities
Up to now, this species has been recorded from Casuarina Beach , in front of the Lizard Island Research Station (material studied here), from Hope Island, North Queensland ( Loch 1989 and pers. comm., material deposited in AMS under the number C.153783) and from Guam ( Carlson & Hoff 2003). A photo record of the Guam species by Carlson and Hoff is available in Rudman (2000b).
Etymology
This species is dedicated to Ken Coles, a great sponsor of the Lizard Island Research Station (LIRS). With his donations, he supports many scientists doing research in the well-equipped laboratories.
Description
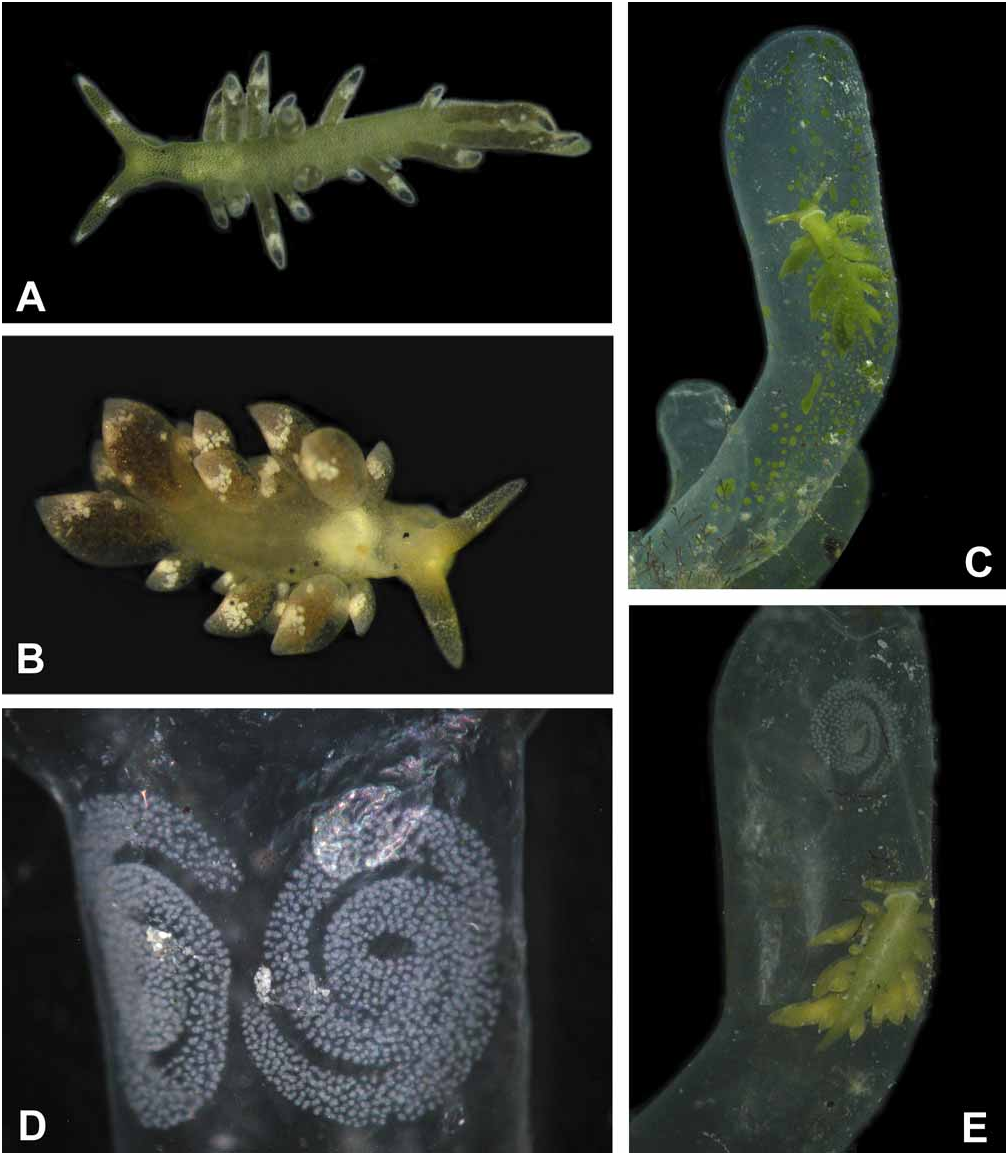
External morphology and colour of living specimens based on five animals ( Figs. 1A–E View FIGURE 1 ): Size from 4 mm up to 6 mm. Body elongate; foot tapering posteriorly; anterior foot without a notch and any propodial tentacles, but slightly extended to lateral sides; rhinophores long, solid and digitiform; eyes lying behind rhinophores on lateral sides; renopericardial prominence inconspicuous; cerata club-shaped to sausage-shaped, in one to two rows, with smaller ones on outer side; cerata not standing very close, those of similar size in opposite position; central notum free of any cerata ( Fig. 1A, 1B View FIGURE 1 ).
Overall colour of body green; under higher magnification, green disintegrating to green dots representing terminal parts of numerous tiny digestive glandular branches. Stripes never present, not even in starving ani- mals ( Fig. 1B View FIGURE 1 ). Rhinophores green with white tips; in median part with a white blotch nearly circling rhinophore. Head completely green, eyes hardly visible. Anterior margin of foot light green to whitish ( Figs.1C, 1E View FIGURE 1 ). Cerata darker green, darkest at apical end; subapical white blotches present, forming an incomplete ring; more patches especially in dorsal areas of cerata. After starving for two to three days, animals showing a more brownish colour and eyes becoming more visible ( Fig. 1B View FIGURE 1 ).
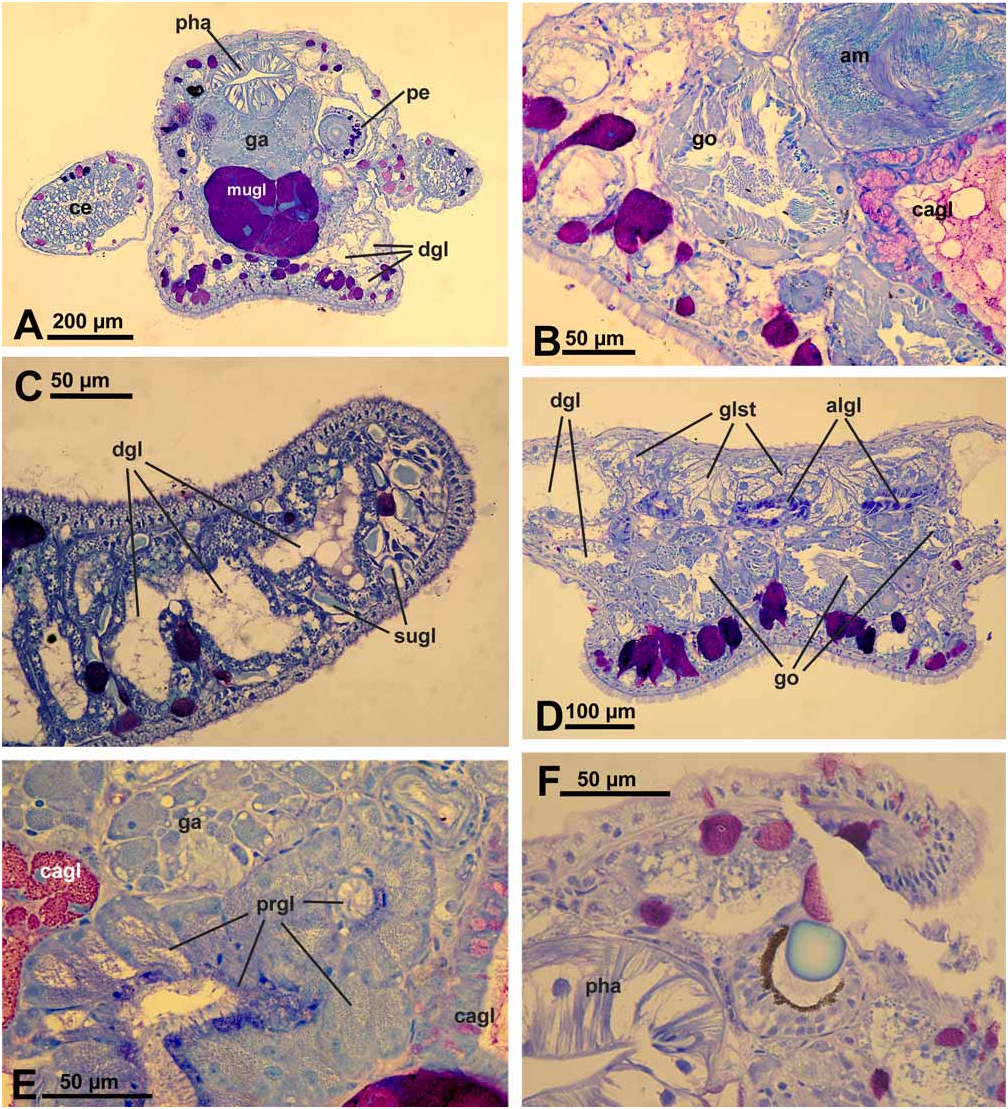
Description of anatomy and histology of preserved specimen ( Figs. 2A–F View FIGURE 2 ).
Digestive tract: Oral tube with ciliated cells, many subepithelial glandular follicles with red-stained mucus surrounding and entering the oral tube. Radula formula 0.1.0. Radula of paratype with 2 teeth in the ascending limb, 7 teeth in the descending limb and 2 preradular teeth ( Fig. 3C View FIGURE 3 ). Teeth sabot-shaped, with smooth edges. Pharynx lined with a cuticle. Short, tube-like salivary glands present; glandular cells with tiny dark bluish stained granules. Oesophagus and stomach lined by a columnar, ciliated epithelium. Digestive gland highly tubulose with many branches running especially beneath epidermis and filling cerata nearly completely ( Fig. 2A View FIGURE 2 ); these diverticula and branches reaching also into rhinophores ( Fig. 2C View FIGURE 2 ) and foot. Intestine with a typhlosolis; epithelium with ciliated cuboidal to columnar cells; distal part of intestine a simple ciliated duct; no glands observable. Anus opening on top of pericardium, middorsally.
Genital system: Gonad lying partly ventral, consisting of follicles with oogonia lying peripheral and spermatogonia in median part ( Fig. 2B View FIGURE 2 ). Ampulla large, with extremely flat epithelium ( Fig. 2B View FIGURE 2 ); sperm in ampulla lying in groups; sperm heads very long, up to 20 µm. Nidamental glands comprising three distinct areas: albumen, capsule and mucus gland. Albumen gland forming several small tubes and running into posterior part of body between gonad and an unidentified gland ( Fig. 2D View FIGURE 2 ). Cells rather broad, apical part filled with violet-staining granules. Capsule gland tubular and mainly situated dorsally to mucus gland. Cells of an intermediate size, i.e., capsule gland cells smaller than mucus cells and larger than albumen gland cells. Capsule gland cells filled with smaller vacuoles staining bright red ( Fig. 2B View FIGURE 2 ). Mucus gland forming a thick tubular structure running ventrally of the pharynx ( Fig. 2A View FIGURE 2 ) and bending back in head area. Cells very large with a red and greasy staining appearance. Presence or absence of receptaculum seminis could not be verified satisfactorily since some slides of that area were lost during preparation. A small bursa copulatrix with rather flat epithelium and very few disintegrating sperm present. Prostate gland composed of large glandular cells with tiny granular appearance and staining light bluish ( Fig. 2E View FIGURE 2 ), forming a rather compact mass behind pharynx. Vas deferens composed of ciliated cells. Penis rather thick, lying within a sheath ( Fig. 2A View FIGURE 2 ). Vas deferens inside of penis with ciliated epithelium, surrounded by musculature. Penis tissue with few subepithelial glandular cells, staining dark violet, in a similar way as observed in body tissue and especially in foot (see below). Distal part of penis with one hollow and curved stylet. Penis opening not observed.
Excretory system: Kidney saclike, situated dorsally above digestive system; epithelium highly folded, with larger cells containing several non-staining vacuoles. Ureter forming a simple ciliated duct. No syrinx present.
Sensory organs: Eyes with pigment cup and round lens. This lens staining homogeneously light blue ( Fig. 2F View FIGURE 2 ). Statocyst large, with one otolith.
Epithelia and glandular structures: Epidermal cells with many vacuoles and very long and densely set cilia especially in anterior part of body and rhinophores ( Fig. 2C View FIGURE 2 ). Tip of rhinophores with larger subepithelial glandular cells having homogeneously light bluish stained contents ( Fig. 2C View FIGURE 2 ).
Subepthelial glandular follicles beneath epidermis of body composed of several large cells. Contents of these cells staining violet, indicating mucus. Cerata epithelium with very flat cells. Few glandular cells staining more homogenously dark violet present subepithelially ( Fig. 2A View FIGURE 2 ). Anterior pedal glands present as subepithelial glandular follicles staining violet to red. Number of these pedal glandular follicles high throughout foot sole.
A special glandular structure starting in middle of body and stretching along whole posterior body part on top of gonad and albumen gland ( Fig. 2D View FIGURE 2 ). Gland composed of follicles with round cells characterized by a large nucleus and large, non- or slightly bluish-staining vacuoles. Neither ducts nor outside opening observed.
Biological notes
Feeding: To observe feeding, two slugs were chosen, which had been kept without algae for four days. Several fresh algal tubes were collected the day before starting the experiment. When these algae were offered, Ercolania kencolesi sp. nov. crawled on top of the first third of one algal tube, sat there for about three to four minutes with the ventral part of the head firmly attached to the alga. In these minutes, the slug pierced the algal cell wall (a process that could not be directly observed) and then started to push the head into the alga. After seven minutes the slug had penetrated completely into the tube. Within the algal tube, it moved immediately to the proximal part of the alga, next to the attachment to the coral rubble. A broad transparent creeping track could be observed, where the chloroplasts were missing. The slug then turned round and crawled to the tip of the algal sac, slurping the chloroplast layer at the inner wall of the alga and leaving a second transparent track. It crawled up and down while slurping the cell sap. The mouth opened regularly for this action. After 45 minutes, 2/3 of the whole sac was sucked out by the slug. After 70 minutes most of the chloroplast layer was consumed. Even after that time the slug still crawled from the bottom to the top, searching for untouched areas within the algal sac. After 80 minutes it stopped searching and was crawling rather lazily. We observed sucking movements now and then, but the slug did not take up any of the small patches of chloroplasts which were still recognizable ( Fig. 1C View FIGURE 1 ). After 18 hours, even those patches were gone ( Fig. 1E View FIGURE 1 ). After consumption of the cell contents, the slug remained in the alga, which did not collapse. Two days after the intrusion, an egg clutch attached to the algal cell wall was observed ( Fig. 1E View FIGURE 1 ). In another experiment, two egg clutches were laid after two and a half days ( Fig. 1D View FIGURE 1 ). In both experiments, the slugs left the algal sac and intruded into another algal tube. These were sucked out immediately in the same way as described above.
Reproduction: For copulation experiments, two animals of similar size (about 5 mm) were chosen. One was taken out of an algal sac; another was sitting outside of Boergesenia . Copulation started with a head to head contact. The rhinophores were touching each other and a few seconds later the animals came to lie side by side with their heads. The penis of one animal was inserted into the body of the other animal. It was not possible to observe whether copulation was reciprocal. This process was observed several times while these animals were kept together for several hours.
Egg lying was observed within the algal sac several times, but also occurred free in the aquaria when no algae were available. The egg mass is a cylindrical tube (up to 1 mm in diameter) coiled anticlockwise into planar spirals of two and a half whorls ( Figs. 1D, 1E View FIGURE 1 ). It is attached to the inner wall of Boergesenia forbesii . Usually one animal laid two egg masses inside the same algal sac. Egg masses measure 4–5 mm in diameter and contain around 500 egg capsules, each with one white egg (size about 100 µm) inside. Capsules are rather spherical and measure around 215µm (measurements taken from 5 eggs).
Development ( Figs. 3A–B View FIGURE 3 ): Spawning took place in July under laboratory conditions. 14 egg masses were observed until their free-swimming larval stage. Egg masses were kept at room temperature (around 20°C) in small vials. Water was changed every two days. Cleavage of eggs happened within several hours and first preveliger were observed after one day. Veliger stage was observed on the 3 rd day after spawning; eyes developed one day later. Shell of preveliger and veliger measured around 130 to 140 µm ( Figs. 3A, 3B View FIGURE 3 ).
After five days, egg masses started to disintegrate and free-swimming larvae were observed within the algal sac. The algal sac disintegrates at the same time; therefore free-swimming larvae escape soon from the alga and tend to swim towards the light. Unfortunately, these larvae were trapped at the water surface and died soon after. Settlement of larvae could not be observed, even in bowls, where Boergesenia forbesii as the food of the adults was offered.
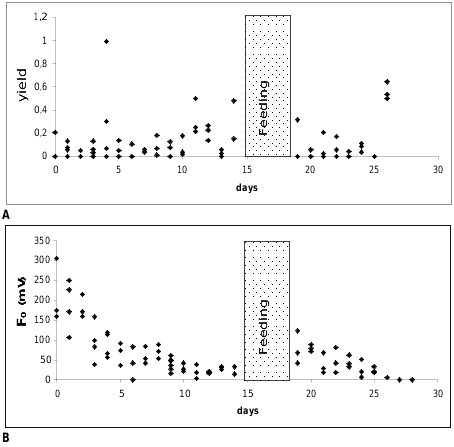
Measurements of the photosynthetic activity ( Figs. 4A–B View FIGURE 4 ): Figure 4A View FIGURE 4 shows the yield values of Ercolania kencolesi sp. nov. (Holotype) plotted versus the number of cultivation days under starving conditions in the aquarium. The yield values of all investigated specimens are on a very low level around 0.1. The ground fluorescence values (F 0 [mV]) plotted against the number of cultivation days under starving conditions in the aquarium decrease quickly from an average of about 150 mV in the beginning to approximately 20 mV after 10 days ( Fig. 4B View FIGURE 4 ).
Starved and pale animals of Ercolania kencolesi sp. nov. with photosynthetic activity approaching zero were allowed to feed again on Boergesenia forbesii for four days. Their green colouration returned and values of their photosynthetic activity (yield) ( Fig. 4A View FIGURE 4 ), as well as ground fluorescence ( Fig. 4B View FIGURE 4 ), increased, but after one to two days of starvation the activity declined to zero again.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |