Schizodactylus salweenensis Dawwrueng, Panitvong, Mooltham, Meebenjamart et Jaitrong, 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4472.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:1385981A-0100-444C-8B74-4E0FAE3E6AE1 |
|
DOI |
https://doi.org/10.5281/zenodo.5958439 |
|
persistent identifier |
https://treatment.plazi.org/id/2451C70D-8F41-FFB3-FF6F-B320FF78F830 |
|
treatment provided by |
Plazi |
|
scientific name |
Schizodactylus salweenensis Dawwrueng, Panitvong, Mooltham, Meebenjamart et Jaitrong |
| status |
sp. nov. |
Schizodactylus salweenensis Dawwrueng, Panitvong, Mooltham, Meebenjamart et Jaitrong View in CoL , sp. nov.
Figs. 1–26 View FIGURES 1–2 View FIGURES 3–9 View FIGURES 10–13 View FIGURES 14–15 View FIGURES 16–21 View FIGURES 22–23 View FIGURES 24–26
Types. Holotype—male; Thailand, Mae Hong Son Prov., Sand dune beside the Salween River, Ban Pala Ue, SobMoei , 1.XII.2015, coll. P. Dawwrueng, K. Mooltham and P. Meebenjamart (THNHM-I-01223, THNHM). Paratype— 5 males (THNHM-I-0 1224 to THNHM-I-01228) , 6 females (THNHM-I-01 229 to THNHM-I-01234), same data as the holotype ( THNHM) ; 2 males (THNHM-I-0 1174 to THNHM-I-01175), 12 females (THNHM-I- 0 1168 to THNHM-I-01 173 and THNHM-I-01176 to THNHM-I-01181), N Thailand, Mae Hong Son Prov., Mae Sariang dist., Huai Umda Beach, Salween River, 15.VI.2017, coll. S. Makchai and C. Chueachat ( THNHM) .
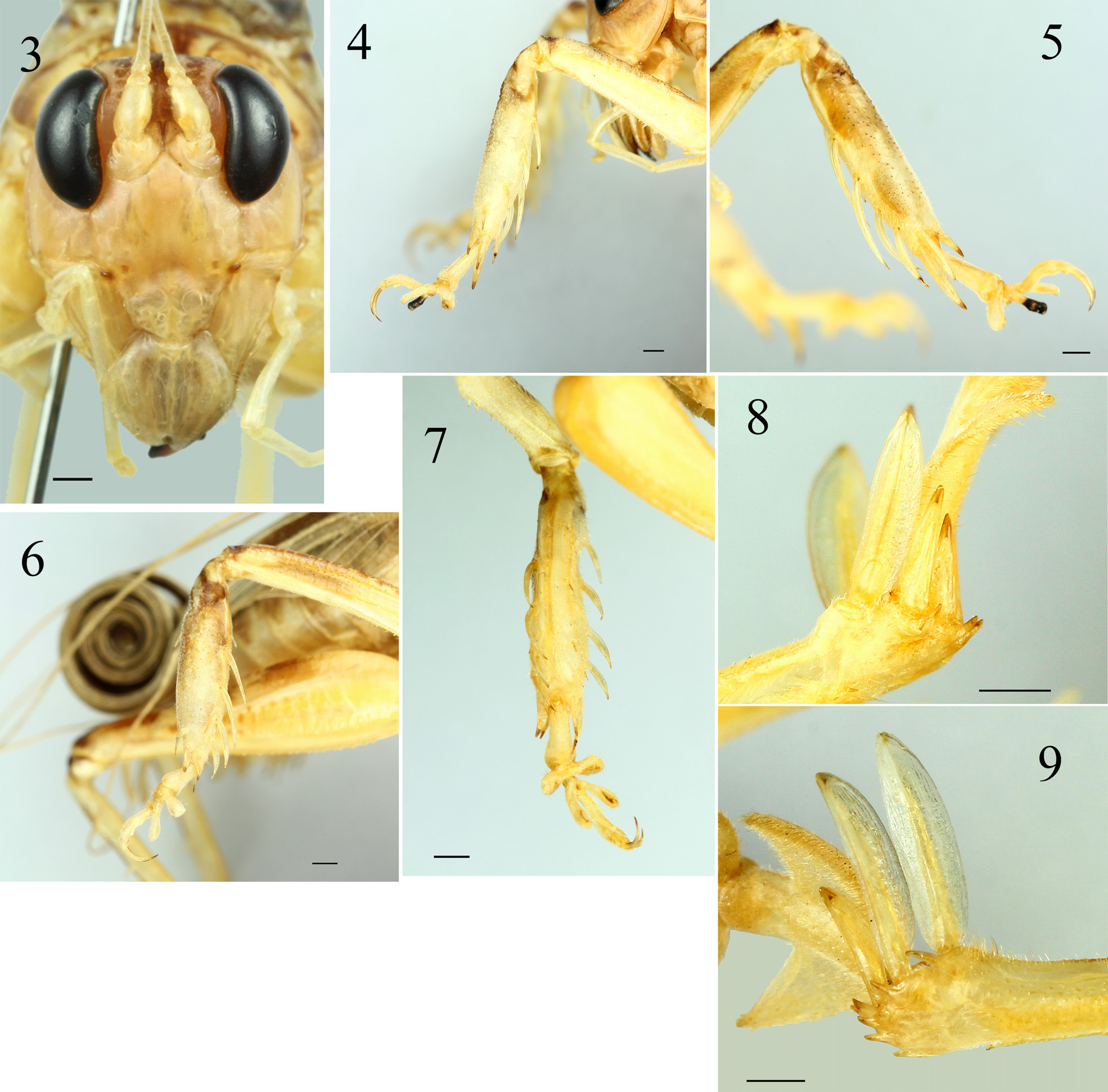
Description. Male ( Holotype and 7 paratypes). Habitus as shown in Figs. 1–2 View FIGURES 1–2 . Body of moderate size. Head ovoid; vertex slightly sloping between compound eye; fastigium verticis small, flat in front view, placed between anterior margin of antenna scrobae ( Fig. 3 View FIGURES 3–9 ). Mandible large and long, concave in middle, with roundly pointed apex, blackish. Pronotum transverse, short, anterior margin almost straight, lateral margin curved, and slightly shallow convex before middle line, posterior margin sinuate and broadly concave, anterior and posterior margin in lateral view raised; transverse sulcus slightly posterior of anterior margin ( Fig. 1 View FIGURES 1–2 ). Tegmen and wings fully developed, apical area rolled ( Fig. 2 View FIGURES 1–2 ). Anterior femur with upper margin finely serrate, outer ventral margin almost straight, with 7–9 inner minute ventral spinules; mesofemur with small tubercles on dorsal surface, with 10–11 minute inner ventral tubercles; postfemur with 32–45 minute spinules on both ventral margins. Anterior and mesotibia swollen as common in members of this genus ( Figs 4–7 View FIGURES 3–9 ). Anterior tibia with 4 elongate inner ventral ( Fig. 4 View FIGURES 3–9 ) and 2 long, 2 short outer ventral spines ( Fig. 5 View FIGURES 3–9 ); 2 apical spurs on both sides, inner spurs longer than outer spurs ( Figs 4–5 View FIGURES 3–9 ). Mesotibia with 4 long outer ventral spines gradually shortened from basal to apical area ( Fig. 6 View FIGURES 3–9 ), and 4 short inner ventral spines, 4 long inner dorsal spines ( Fig. 7 View FIGURES 3–9 ) and one short outer dorsal spine near apical spurs ( Fig. 6 View FIGURES 3–9 ); 2 apical spurs on both sides, inner spurs longer than outer spurs. Posttibia with 3–4 outer dorsal and 4 inner dorsal spines; with 3 apical spurs at both side, inner spurs longer than outer spurs; the first outer spur lanceolate, not very broad with subacute apex, the second and third outer spurs smaller, thinner than the first spur, their shape triangular gradually narrowing to acute apex, third outer spur with acute apex, sharper than second outer spur, both unequal in size ( Fig. 8 View FIGURES 3–9 ); first inner spur lanceolate, slightly broader than the second, but of same length, apex roundly subacute, second inner spur with lower margin slightly concave, subacute apex and more sharpened than the first, third inner spur of similar shape as second but distinctly smaller, about half as long, with apex more sharpened than the second ( Fig. 9 View FIGURES 3–9 ); ventro-apical margin of posttibia with 4 short spines and with 1 longer spine at internal angle. Tarsi of all legs with 4 segments and a pair of elongate apical claws, second and third segments very short with a pair of large plantulae, plantulae of second segment wider and larger than plantulae of third segment; first segment of hind tarsus with a pair of large triangular plate. Tenth abdominal tergite transverse, interrupted in middle. Epiproct with widened lateral expansions ( Fig. 10 View FIGURES 10–13 ), medial areas of hind margin slightly concave as truncate shape that is deeply furrowed in midline ( Figs 11–12 View FIGURES 10–13 ), the lateral expansion slightly longer than medial areas, the upper surface is covered by dense small granulae ( Fig. 12 View FIGURES 10–13 ), with obliquely water drop shape with rounded apex projection, furrowed in midline from basal running through the projection ( Figs 11–12 View FIGURES 10–13 ). Paraproctes with a narrow projection terminating into an acute tooth ( Figs 10–11 View FIGURES 10–13 ), protruding from underneath subgenital plate ( Fig. 10 View FIGURES 10–13 ). Subgenital plate 1.46–1.48 times broader than long, gradually triangular with a lobe at apex that is furrowed in midline forming a pair of lobes, upper margin concave triangular excised and lower margin concave, this extension broader than long and less rounded ( Fig. 10 View FIGURES 10–13 ), apex deeply triangularly excised in posterior view ( Figs 11–12 View FIGURES 10–13 ), posteriorly truncate and protruding downward in lateral view ( Fig. 13 View FIGURES 10–13 ), pubescent especially the extension. Cerci elongate and rounded, unsegmented, slightly curved, medial area narrower than basal and apical areas, ventral side of median to apical area furrowed in mid line, apex slightly compressed and subacute.
Female ( Paratype). Habitus as shown in Figs. 14–15 View FIGURES 14–15 . General habitus as in male. Apical spurs of posttibia similar as in male ( Figs 17–18 View FIGURES 16–21 ). Tenth abdominal tergite short, transverse, subfused with epiproct. Epiproct subtriangular, furrowed in midline, apex rounded ( Fig. 19 View FIGURES 16–21 ). Paraproctes large, triangular with subacute apex, easily visible in ventral and posterior view ( Fig. 20 View FIGURES 16–21 ). Subgenital plate simple, broader than long with broadly rounded apex ( Fig. 20 View FIGURES 16–21 ). Ovipositor very small and short, but clearly protruding from the subgenital plate, slightly downward at apex in lateral view ( Fig. 21 View FIGURES 16–21 ). Cerci elongate but shorter than in male, narrower in apical two thirds and compressed.
Coloration. Generally whitish brown ( Figs 22–23 View FIGURES 22–23 ). When alive, compound eyes dark brown, vertex with 5 interrupted dark brown longitudinal bands, fastigium verticis brown. Pronotum with dark brown adornment and blackish posterior of lateral lobe. Wings blackish with bright venation. Tegmen dorsally near base brown with yellowish venation, remainder of dorsal surface yellowish transparent, humeral angle dark brown, upper area of lateral field to apex of tegmen with brown venation and smoky grey cells, remainder transparent. Anterior tibia with brown spots on dorso-internal margin; mesofemur with brown spots on dorso-external margin and a brown band band below; postfemur with 3 dark brown bands on dorso-external surface and large brown spots in apical area of lateral surface, around and including these areas with dark brown dots. All knees dark brown. Anterior and mesotibia with brown spot at base, remaining area stained by greyish ornament. Posttibia with genicular area dark brown. Armament of all tibia whitish brown with dark brown apex. Tarsi whitish brown. Cerci whitish brown.
Measurement (mm). Body 26.9–27.2 (male), 27.9–28.3 (female); pronotum in middle 2.7–2.9 (male), 3.0–3.2 (female); pronotum at the sides 3.6–3.9 (male), 3.6–4.0 (female); pronotum width 7.8–8.3 (male), 8.4–9.1 (female); anterior femur 9.3–9.7 (male), 9.4–9.9 (female); mesofemur 10.6–11.0 (male), 10.6–11.3 (female); postfemur 17.1–17.8 (male), 17.2–17.9 (female); posttibia 14.0–14.4 (male), 14.1–14.9 (female); ovipositor 0.3–0.4; cercus 7.8–8.4 (male), 5.3–5.8 (female).
Distibution. Sand beach along Salween River, Mae Hong Son Province, Thailand.
Diagnosis. The new species is most similar in size and the apical area of the subgenital plate to S. burmanus Uvarov, 1935 and S. tuberculatus Ander, 1938 . However, S. salweenensis can be distinguished from S. burmanus by the following characters: male subgenital plate broader than long, the apical area of the subgenital plate transverse narrow, broader than long and furrowed in mid line similar to a pair of lobes, while the subgenital plate is longer than broad and the extension is rounded with flat top in vental view in S. burmanus ; the first internal spur slender, the second less concave, and the third clearly smaller than the second, about half as long in the new species, while in S. burmanus , the first internal spur is broader, the second is clearly concave, and the third is slightly shorter than the second; the shape of the female subgenital plate more broadly rounded in S. salweenensis than in S. burmanus . Schizodactylus salweenensis is easily separated from S. tuberculatus by comparing the apical area of the male subgenital plate as described above and by the shape of the internal spurs of the posttibia. In contrast, in S. tuberculatus , the apical area appears like a pair of knobs protruding forward and the posttibial apical spurs are similar to those of S. burmanus , in which the shape of the first and the second internal spurs are of similar size. From the remaining species of the genus, the new species differs mainly by the shape of the subgenital plate and the shape of the apical spurs of the posttibia.
Etymology. The new species is named after the Salween River where it was collected. We would also like to propose the English common name as Salween Dune Cricket and the Thai common name as ???????????????????????.
Biology. The new species can be found living along the sand banks of the Salween river ( Fig. 24 View FIGURES 24–26 ). In February of 2018, during the dry season, when the second author (NP) visited the habitat, beach and dunes were common along the river bank varying in length, depth and height from the river surface. We surveyed a part of the river 1 kilometer long and 50–200 meters wide. The slope on the bank varied greatly from 90 degrees to no slope at all. According to the local residents, the majority of the beach would be inundated during the monsoon season. At night, the dune crickets can be easily located by looking for their newly dug burrows ( Fig. 25 View FIGURES 24–26 ), where the wet sand from their burrows is of different color from that on the surface. The burrows were mostly found close to the water line, where the sand under the surface still retained some moisture and is thus suitable to form stable burrows. The closest burrows were about 1.5 meters and the farthest about 5 meters from the water line. The length of a burrow was around 50–60 cm deep. The crickets were mostly found at the entrance of the burrows with their head protruding and their antennae sweeping back and forth. To dig, Individuals use the hind legs to kick out the sand while facing into the burrow. If disturbed, the cricket quickly retreats into the burrow and uses some sand to cover the hole. However, if they were spotted away from the burrow, they tend to remain motionless probably relying upon their camouflage to escape detection. During our surveys, a few small moths were found on the bank but no predation by the crickets was observed. We believe that as more plants start growing on the dunes during the dry season, more prey would be available for the crickets. Potential predators of the crickets include small frogs ( Fejervarya sp.), which we found in burrows and small carnivores of which we found a set of foot prints. This was most probably of civet. We were able to locate a few unused burrows during the day but most of the burrows, which we observed during the night were closed and the crickets were hidden under the sand during the day. According to the locals, the cricket can be found only in dunes of low disturbance. Most of the crickets that we observed in February were in nymphal stages. The locals said that they would be fully grown in May–June period, which coincides with the beginning of the monsoon season in the region. This statement is most likely true because we collected adult specimens in July. Adults can be found until December when the authors (PD, KM and PM) found adults sharing the habitat with nymphs in the same areas ( Fig. 26 View FIGURES 24–26 ).
Currently a few dam projects, both up and downstream from the type locality, are being proposed by Myanmar government. The dam(s), if built, would inundate the river bank habitat of the new species. Furthermore, the river downstream of the dam will experience unnatural water flow which would disturb the sand beach/dunes formations. This in turn will adversely affect the habitat of this unique species. The dam(s) are also likely to create unnaturally high fluctuations of water levels. This might directly harm the crickets living in burrows along the sand banks.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Ensifera |
|
Family |
|
|
SubFamily |
Schizodactylinae |
|
Genus |