Tephritis arnicae ( Linnaeus, 1758 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4007.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:FDA8C432-B634-4CC9-9332-6D24D82C163E |
|
DOI |
https://doi.org/10.5281/zenodo.6110799 |
|
persistent identifier |
https://treatment.plazi.org/id/25512715-FFAF-F97A-FF1B-0C2DFEF1FC61 |
|
treatment provided by |
Plazi |
|
scientific name |
Tephritis arnicae ( Linnaeus, 1758 ) |
| status |
|
Tephritis arnicae ( Linnaeus, 1758) View in CoL
Figs. 3–4 View FIGURES 1 – 4 , 7–8 View FIGURES 5 – 8 , 14–18 View FIGURES 9 – 18. 9 – 13 , 19–22 View FIGURES 19 – 22. 19, T .
Musca arnicae Linnaeus 1758: 600 View Cited Treatment ; 1761: 460; 1767: 997.
Trypeta arnicae: Meigen 1826: 333 .
Tephritis arnicae: Latreille, 1804: 335 View in CoL ; Zetterstedt 1847: 2213; Loew 1862: 99; Schiner 1864: 165; Hendel 1927: 182; Séguy 1934: 156; Hering, 1944: 20; Mihályi 1960: 66; Richter 1970: 165; Kabos & Aartsen 1984: 28; Foote 1984: 127; Janzon 1985: 102; White 1987: 101; Nowakowski 1991: 178; Belcari, Girolami & Rivosecchi 1995: 11; Merz 1994: 63; 1999, 2001 а, 2001 b; Bjureke & Greve 1996; Norrbom et al. 1999: 214; Korneyev 2003: 11; Pakalniškis et al. 2006: 87; Heřman & Kinkorová 2009; Smit 2010: 108; Korneyev 2011: 5.
Synonyms:
Trypeta flavicauda Meigen 1826: 336 .
Trypeta arnicivora Loew 1844: 384 .
Trypeta eggeri Frauenfeld 1857: 544 ; 1864: 164.
Tephritis arnicae eggeri: Hering 1944: 20 .
Tephritis melanotrichota Hendel 1903: 383 View in CoL ; Hering 1944: 20; 1947: 7. Tephritis malanotrichota: Becker 1905: 139 View in CoL (misspeling).
Type material. Lectotype (sex not stated) of Musca arnicae : specimen illustrated by Aldrovandi (1602) ( Fig. 19 View FIGURES 19 – 22. 19, T ), which Linnaeus referred to in his description, “Habitat in discis florum radiatorum, imprimes Arnices montanae ”, designated by White (1987); the material lost. [The specimen in Linnaeus’ collection labelled as M. arnicae actually is a specimen of Xyphosia miliaria (Schrank 1781) ( White 1987) .]
Examined type specimens: Syntype Trypeta flavicauda : 1♀, [probably Germany: [Stolberg] “ flavicauda, Meigen ” ( MNHN) ( Fig. 20 View FIGURES 19 – 22. 19, T );
Possible syntypes Trypeta arnicivora : 2♀, Poland: 2♀ with small square labels “Siles[ia]” and “coll. H. Loew” ( ZMB).
Syntype 1♀, Trypeta eggeri : Austria: “Doron. pard alpick”, “ eggeri det. Frauenfeld” ( NHMW) ( Fig. 21 View FIGURES 19 – 22. 19, T ); possible syntypes: 1♂: “Frauenf”, “ eggeri / Frnfld.”, “coll. H. Loew”; 3♂: ex Doroni[cum] /co Egger” [two on same type of pins without label following this specimen], “coll. Loew”.
Syntype ♀ Tephritis melanotrichota : “ Norway: Erfjord (Strand.)”( Fig. 22 View FIGURES 19 – 22. 19, T ).
The status and number of type specimens of nominal species now considered synonyms of T. arnicae is reconsidered here. Type specimens of the synonyms were examined. The only existing type female of T. flavicauda Meigen is considered here a syntype, as origally Meigen (1826: 337) mentions that it was reared “aus Maden die in den Samen des gemeinen Wolverleies ( Arnica montana ) lebten” [from maggots living in the seeds of Arnica montana ] ( i.e., there could have been more than one specimen originally). Syntypes of Trypeta arnicivora Loew from von Heyden’s collection, apparently as well as the specimens from Schilling’s collection are apparently lost; 2♂ with small labels “Siles.” are not evidently the speimens, collected by Scholtz in 1858, and are possible syntypes of T. arnicivora .
Georg Ritter Frauenfeld (1857) noticed that Trypeta eggeri ( Fig. 21 View FIGURES 19 – 22. 19, T ) is reared from Doronicum pardalianches and he distinguishes T. eggeri and T. arnicivora from Arnica montana as different species.
Type of T. melanotrichota ( Fig. 22 View FIGURES 19 – 22. 19, T ) is described from Norway and has unusual wing pattern for T. arnicae, Hendel noticed the similarity of T. melanotrichota and T. arnicae in wing pattern and coloration of abdominal setulae, but he turns much attention to isolated apical spots as the good character for a new species.
Non-type material examined. Austria: Manhartsberg, coll. Mehr., 18 (sex unknown), 1♀ (A. Siebeck) ( ZMB); Kärnten, Mallnitz, 16.06– 13.07.1931, 67♂, 69♀ (M. Ude) ( ZMB); Germany: Flinsberg, Prov. Brandenbg., coll. Mehr, 1892, 1♂, 6♀ ( ZMB); “Guestphal.” (Westfalia), “2534”, 1♀ (Suffrian) ( ZMB); Bavaria, mer. Ammergauer Bergem Frieder-Gebiet, 1200 m, 23.07.1948, 1♀ (F. Pechschnait); Bavaria, Siegesdorf, 20.04.1959, 17♂, 9♀ (H. Freude); Stammbach, Ofr., 3.07.1963, 1♀ (S. Vierling) ( ZSSM); Hungary (?): “ Ungarn ”, 1♀ (coll. Mehr) ( ZMB); Italy: Macugnaga, 24.07.1900, 2♂, 2♀ (Oldenberg) ( ZMB); Moldova: Kotovsk, 6 km NW Loganeshstski forest, ex Doronicum , coll. 16.06.1987, em. 24.06.1987, 6♀ (Korneyev) ( SIZK); Poland: “Liegnitz” (Legnice), 2♂, 3♀; “Silesia, Initio mensis Augusti 1858, caulibus inflatis Doronici austriaci ”, 12♂, 11♀ (Scholtz), “Silesia, Scholtz”, 3 ♀ (“Coll. H. Loew”) ( ZMB); Sweden: “Zttst.” [Zetterstedt], “ex Arnic.”, “arni- /cae / Lin.”, “coll. H. Loew”, 1♂; “Zetters”, “coll. H. Loew”, 1♀ and following 1♂, 1♀ (coll. H. Loew) on imilar pins ( ZMB); Switzerland: Simplon, EL, Hosoix, 21– 27.07.1984, 23♂, 24♀ (G. R. Langohr) ( RMNH); GR, 1730 m, Ftan, ex Arnica montana , coll. 15.08.1988, ep. 24.08.1988, 1♀ (Merz) ( SIZK); GR, 2220 m, Ftan, ex Arnica montana , coll. 18.08.1988, ep. 1.09.1988, 1♂ (Merz) ( SIZK); GR, 1600 m, Lenzerheide, ex Arnica montana , 8.08.1992, 1♂, 1♀ (Merz) ( SIZK) GR, 1600 m, Lenzerheide, ex Arnica montana , 8.08.1992, 1♀ (Merz) ( ZISP); Ukraine: Hoverla mt., h=850 м, 17.07.2011, from stem galls of the Doronicum austriacum , exit 22.07.— 3.08.2011, 13♂, 10♀ (S. & V. Korneyev) ( SIZK).
Diagnosis. Tephritis arnicae can be easily recognized from other Palearctic Tephritis species by the following unique combination of characters: wing pattern reticulate and dark, including anal lobe, posterior orbital and posterior notopleural setae black, and setulae on the thorax and abdominal tergites at least partly black.
Redescription.
Head ( Fig. 7 View FIGURES 5 – 8 ) shape as in most other Tephritis . Length: height: width ratio = 1:1.1:1.54. Frons subquadrate, twice as wide as eye. Compound eye 1.3 times as high as long. Gena 0.55 times as high as length of flagellomere 1. First flagellomere 1.6 times as long as wide. Coloration yellow to brown, mostly whitish microtrichose, with black ocellar triangle and medial part of occiput. Flagellomere 1 dark yellow, arista dark brown. Genal setulae black.
Ocellar, medial vertical, anterior and posterior orbital and frontal setae black and acuminate; genal seta black and acuminate; remaining setae lanceolate whitish or yellowish. Postocular setae black and white, setulae black
Thorax ( Fig. 7 View FIGURES 5 – 8 ): Ground color predominantly black; postpronotum, small dorsal part of anepisternum and scutellum laterally dark yellow. Thorax densely microtrichose, microtrichia gray to ochreous yellow. Most of the setae black, including posterior notopleural seta. Setulae on mesonotum mainly black, with a few white ones; scutellum with several white marginal setulae on each side. Calypteres white. Halter yellow.
Legs ( Fig. 7 View FIGURES 5 – 8 ): Dark yellow; fore femur with 2 rows of white posterodorsal and one row of brown posteroventral setae; mid and hind legs with brown setae and setulae.
Wing ( Figs. 4 View FIGURES 1 – 4 ): pattern dark with numerous hyaline spots and dots. Cells bm and bcu hyaline; cell c with dark bar at base and another at middle of cell. Pterostigma brown, with single subapical hyaline spot. Cell r1 brown with single hyaline dot posterior to pterostigma; with 2 large marginal hyaline spots separated by narrow brown bar, proximal spot twice as wide as distal; and with small subapical hyaline spot. Cell r2+3 hyaline at base, with dark area posterior to pterostigma; with three hyaline spots posterior to spots in r1 separated by narrow dark bars, or often partly merged, two proximal spots much larger than distal spot; preapical brown area (posterior to cell r1 apex) wide and usually with two hyaline spots, one posterior to apex of vein R2+3, and one before apex of cell. Cell br mostly dark except a hyaline basal spot posterior to cell c and several (4–7) smaller hyaline dots in distal part. Crossvein r–m surrounded by 4 hyaline spots rarely merged into hyaline lines. Cell r4+5 with basal one-third anterior to crossvein dm-cu with two hyaline spots, usually separated, posterior spot three to four times larger than anterior one; middle third of cell brown, with 3–8 round hyaline dots; subapical area of cell with two wide dark spots at apices of R4+5 and M usually connected (sometimes one of apical spots can be separated) to each other and remaining dark pattern by a narrow “peduncle”, and forming wide “apical fork”. Cell m dark, with 10–15 hyaline spots of various sizes. Cell dm mostly dark, with more than a dozen small hyaline spots and one large pear or 8- shaped hyaline spot near r-m level. Cell cu dark with 10–14 round hyaline spots of various sizes, either separate or partly fused. Anal cell dark with 4–5 round hyaline dots. Anal lobe dark with 4–5 hyaline spots, separate or fused. Alula usually with dark pattern.
Abdomen ( Fig. 8 View FIGURES 5 – 8 ): Black, tergites entirely microtrichose, mostly with black setulae (white setulae on the first abdominal tergite) and white marginal setae.
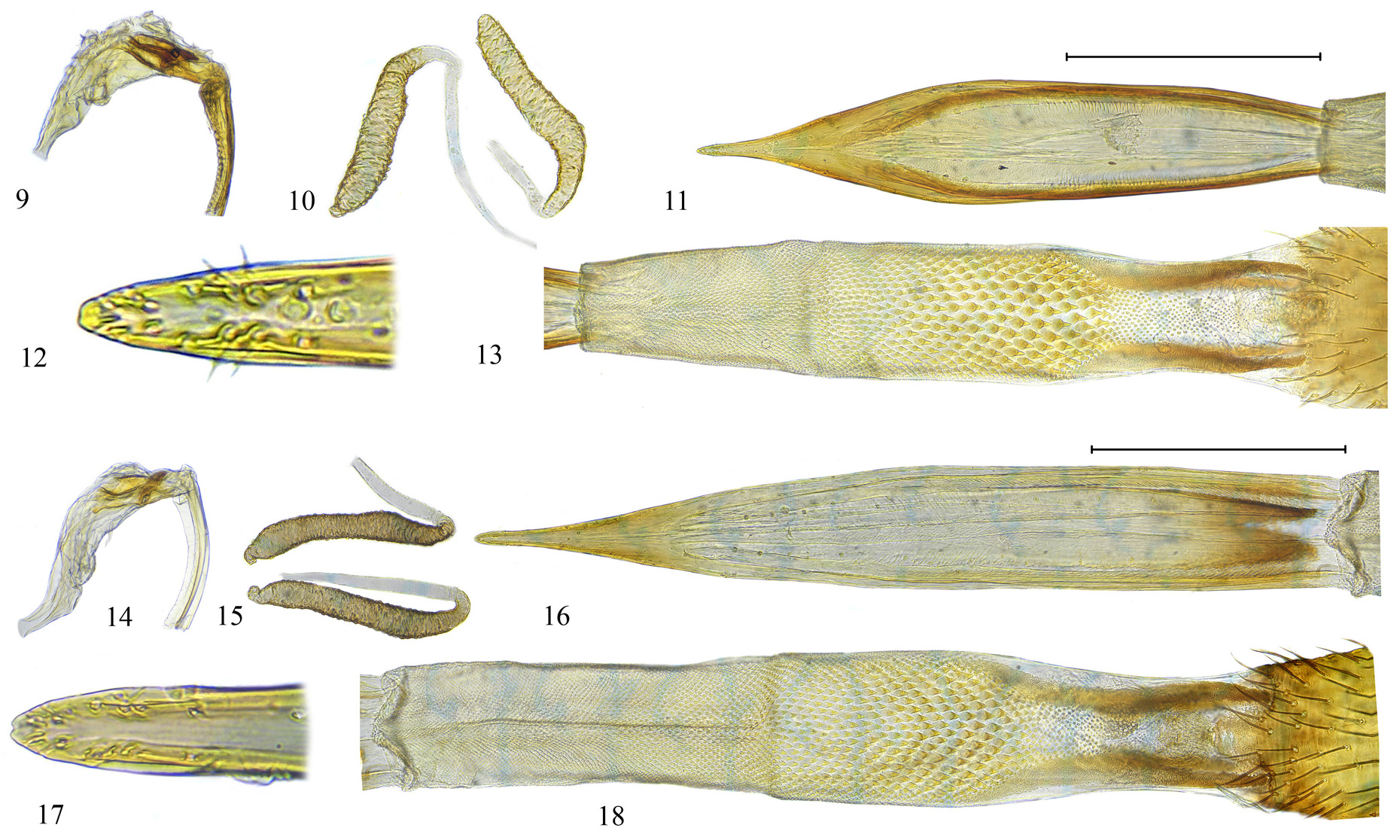
Genitalia. Male. Glans of phallus ( Fig. 14 View FIGURES 9 – 18. 9 – 13 ) moderately short, mostly membranous with apical gonopore, without dorsal tale-like process. Preglans without spines.
Female. Oviscape dark yellow, entirely black setulose; as long as 3 last abdominal tergites combined ( Fig. 2 View FIGURES 1 – 4 ). Aculeus brown, 7 times as long as wide, apex blunt, insignificantly incised ( Figs. 16–17 View FIGURES 9 – 18. 9 – 13 ). Eversible membrane with two pairs of taeniae 0.25 times as long as membrane itself; on ventral side membrane with scales of different sizes, the largest triangular and sharp ( Fig. 18 View FIGURES 9 – 18. 9 – 13 ). Spermathecae long and narrow, 8.5 times as long as wide ( Fig. 15 View FIGURES 9 – 18. 9 – 13 ).
Measurements. Female. Body length 5.5–6.1 mm, wing length 4.5–5.0 mm, aculeus length 1.65–1.87 mm. Male. Body length 4.0– 4.5 mm, wing length 3.9–4.6 mm.
Host plants. Larvae develop in flowerheads of Arnica montana L., Doronicum grandiflorum Lam. , D. austriacum Jacq. , and D. hungaricum Reichenb. fil. [references?] One to three larvae were found in flower heads of early flowering D. hungaricum in Moldova (V. Korneyev, pers. comm.). In Ukraine adults were reared from stem galls of D. austriacum ; larvae apparently moved from the flowerhead into the stem through the peduncle to induce a stem gall ( Fig. 26 View FIGURES 23 – 26. 23 ). In Arnica montana , one to twelve larvae were found feeding in one flowerhead in Sweden ( Janzon 1985). The character of larval feeding in other host plants is unknown. References to Aster bellidlastrum as a host plant are believed to have been based on misidentifications (see Hendel 1927).
Distribution. Austria ( Schiner 1864), Andorra ( Merz 2001b), Belgium ( Baugnée 2006), Bulgaria ( Drensky 1943), Czech Republic ( Kinkorová 1997; Heřman & Kinkorová 2009), Denmark ( Zetterstedt 1847), France ( Séguy 1934), Germany ( Merz 1999), Hungary ( Mihályi 1960; Merz 2001a), Italy ( Belcari et al. 1995), Moldova ( Korneyev 2003), Norway ( Hendel 1903), Lithuania ( Pakalniškis et al. 2006), Poland ( Nowakowski 1991), The Netherlands ( Kabos & Aartsen 1984; Smit 2010); Slovakia ( Kinkorová 1997; Heřman & Kinkorová 2009), Sweden ( Linnaeus 1758; Zetterstedt 1847; Janzon 1985), Spain ( Merz 2001b), Switzerland ( Merz 1994), Ukraine ( Korneyev 2011).
Remarks. The wing pattern of T. arnicae shows conspicuous variability due to the variable size of hyaline spots that may be either partly fused or entirely isolated; these include round dots bordering r-m, which may be fused to form hyaline bars, as well as larger spots in cells r1, r2+3, m and cu which are partly fused as in the female syntype specimen of T. melanotrichota , and sometimes the dark spot at the apex of R4+5 which may be isolated from the rest of the wing pattern ( Fig. 22 View FIGURES 19 – 22. 19, T ).
Discussion. Tephritis arnicae and T. arsenii apparently form a monophyletic lineage in the genus Tephritis based on quite uncommon characters for genus, such as dark areas on the anal lobe and anal cell, large spots at the apices of veins R4+5 and M, and dark setulose abdominal tergites; but all of these character states occur separately elsewhere in other species of the genus and none is a unique synapomorphy. However, their close relationship is supported by the larval feeding in the closely related host plants of the tribe Senecioneae. No other Palearctic species of the genus are using these plants as hosts, so this fact supports the hypothesis of their close relationships, along with general similarity and presence of the mentioned possible synapomorphies. Tephritis arnicae and T. arsenii occur in moderately humid mountainous areas of Europe (including Scandinavia) and the Middle East, respectively. Their distributional ranges appear to be isolated. Despite the special studies of the Caucasian fauna in 2013 and thorough analysis of collections in 2013-2014, including vast material from the former Soviet Union in the Zoological Institute, St. Petersburg, there is a gap between their distributional ranges, which consists of a large East European Plain and Eastern Mediterranean region (southern Balkan Peninsula and western Asia Minor). If T. arnicae and T. arsenii are sister species, we hypothesize that their isolation resulted from a primarily continual range of their ancestral species during the Pleistocene glaciation, split by the Holocene xerothermic period and sea transgressions into the Aegean Land. More detailed data on the relationships, including molecular data, is in preparation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tephritis arnicae ( Linnaeus, 1758 )
| Korneyev, Severyn V., Khaghaninia, Samad, Namin, Saeed Mohamadzade & Zarghani, Ebrahim 2015 |
Tephritis arnicae eggeri :
| Hering 1944: 20 |
Tephritis melanotrichota
| Hering 1944: 20 |
| Becker 1905: 139 |
| Hendel 1903: 383 |
Trypeta eggeri
| Frauenfeld 1857: 544 |
Trypeta arnicivora
| Loew 1844: 384 |
Trypeta arnicae :
| Meigen 1826: 333 |
Trypeta flavicauda
| Meigen 1826: 336 |
Tephritis arnicae :
| Korneyev 2011: 5 |
| Smit 2010: 108 |
| Pakalniskis 2006: 87 |
| Korneyev 2003: 11 |
| Belcari 1995: 11 |
| Merz 1994: 63 |
| Nowakowski 1991: 178 |
| White 1987: 101 |
| Janzon 1985: 102 |
| Kabos 1984: 28 |
| Foote 1984: 127 |
| Richter 1970: 165 |
| Mihalyi 1960: 66 |
| Hering 1944: 20 |
| Seguy 1934: 156 |
| Hendel 1927: 182 |
| Schiner 1864: 165 |
| Loew 1862: 99 |
| Zetterstedt 1847: 2213 |
| Latreille 1804: 335 |