Holothuria (Semperothuria) roseomaculata, Kerr, Alexander M., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3641.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:98BA29AC-6EE5-43E0-8464-D07E845E75D8 |
|
DOI |
https://doi.org/10.5281/zenodo.5673167 |
|
persistent identifier |
https://treatment.plazi.org/id/260587D9-FFC3-FF91-FF68-FA8BFFB0FB3A |
|
treatment provided by |
Plazi |
|
scientific name |
Holothuria (Semperothuria) roseomaculata |
| status |
sp. nov. |
Holothuria (Semperothuria) roseomaculata n. sp.
( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Holothuria flavomaculata, Yamanouti 1939: 604 –634; Yamonouchi 1956: 361; Amesbury et al. 1977: 14–16; Grosenbaugh 1981: 51–53; Colin & Arneson 1995: 260, fig. 1232; Buckius et al. 2010: 964, 966–967.
Holothuria (Semperothuria) flavomaculata, Cherbonnier 1980: 634 –635, fig. 10; Féral & Cherbonnier 1986: 90–91, fig. 40 D, unnumbered fig. (p. 90); Conand 1989: 27 et non seq., figs. 18–19; Purcell et al. 2012: 50–51 and unnumbered figs.
Holothuria (Semperothuria) sp., Kerr et al. 2007: 15, 31, fig. 3b; Kim et al. 2013: in the press.
Holothuria (Semperothuria) non flavomaculata , Friedman et al. 2008: 8, 12; Tardy & Pakoa 2009: 10, 49–50.
Non Holothuria flavomaculata, Semper 1868: 87 , 277, pl. 30, fig. 26.
Material examined. Type material housed at FLMNH. Holotype: UF 5850, O'Keefe's Island, Yap, FSM, 9.5136° N, 138.1318° W, seagrass and mangrove fringing reef, 0–1 m depth, colls. K. Netchy and A. M. Kerr, 30.VII.2007, GenBank nucleotide sequences JN207626 View Materials and JN207532 View Materials (see Honey-Escandón et al. 2012).
Paratype: UF 5872, O'Keefe's Island, Yap, FSM, 9.5136° N, 138.1318° W, seagrass, 0–1 m depth, colls. K. Netchy and A. M. Kerr, 30.VII.2007, GenBank nucleotide sequences JN207627 View Materials and JN207542 View Materials (see Honey- Escandón et al. 2012).
Other material: USNM E25730, Bougainville Island, Bismarck Archipelago, PNG, coll. International Indian Ocean Expedition aboard the R/V Te Vega, 10.IX.1963. USNM E16766, Station Yap-69, Yap, FSM, coll. R. W. Hiatt, 1950. USNM E16773, Station Yap-141, Yap, FSM, coll. R. W. Hiatt, 1950. UGI 2408 Tomil Harbor, Colonia, Yap, patch reef with Acropora corals, approx. 2 m depth, coll. D. A. Grosenbaugh, 9.VII.1977. UF 1341, Omodes Channel, Iwayama Bay, Palau, 1 m depth, coll. G. Paulay, 14.III.1996. UF 2322, Lenger Island, Pohnpei, FSM, 2 m depth, coll. J. Starmer, 13.III.2003. UF 11359, Weno, Chuuk, FSM, 0 m depth, coll. S. Kim, 11.VII.2011. UGI 6682, First lagoonal patch reef due west of harbour, Malakal, Palau, 4 m depth, coll. A. M. Kerr, 1.III.1992. UF 11294, Station 1, Outside Colonia Harbor, Yap, FSM, 1 m depth, coll. E. Tardy, 25.IX.2009.
Comparative Holothuria (S.) flavomaculata material: YPM IZ007026, Yale Seychelles Expedition Station 35B, Moyenne Island, Seychelles Islands, coll. A. J. Kohn, 20.I.1958. USNM E51765 View Materials , Piti Bay, Guam, Micronesia, 3 m depth, coll. A. M. Kerr, VIII.1998. UGI 5722, Piti Bay, Guam, USA, 7 m depth, coll. A. M. Kerr, 7.VII.1991. UF 335, Rangiroa Atoll, Tuamotu Islands, French Polynesia, 3 m depth, coll. G. Paulay, 1.X.2001. UF 4363, Erakor Lagoon, Efate Island, Shefa, Vanuatu, 0–1 m depth, coll. C. Meyer, 3.I.2005. UF 6331, Chez Go, Saint-Gilles, Reunion Island, 0–1 m depth, colls. N. Hubert & F. Michonneau, 24.VII.2007. UF 1353, Hekili Point, Maui, Hawaii, USA, 0–1 m depth, coll. C. Pittman, 8.XI.1998. UF 1857, Off Alofi Wharf, Niue Island, outer reef slope, 14 m depth, coll. G. Paulay, 18.V.1986. UF 6249, French Frigate Shoals, Hawaii, USA, 31 m depth, colls. G. Paulay et al., 23.X.2006. UF 994, near Oneroa Landing, Mangaia, Cook Islands, depth unrecorded, coll. G. Paulay, 18.XI.1984.
Diagnosis. Dorsal papillae rose-coloured, their distal extremities white.
Description. External appearance: Live in situ length and mid-body width of holotype 14 cm and 6 cm, respectively, in preservative 10 cm by 3 cm. Live in situ lengths of 31 uncollected specimens from the type locality with mean and median of 20 cm, standard deviation of 5 cm, and range of 12–34 cm. Lengths of five randomly selected preserved specimens from various sites including the type locality with mean of 16 cm, median of 14 cm, standard deviation of 8 cm, range of 9–25 cm. Body wall of living specimens usually deep reddish brown, fading to medium grey in preserved specimens ( Fig. 1 View FIGURE 1 B). Base of papillae salmon pink in life ( Fig. 1 View FIGURE 1 F), quickly fading to off-white in alcohol. Distal extremities of papillae white in life, but white sometimes not visible when papillae partially or wholly contracted. Body vermiform, tapering slightly anteriorly, body wall thin, smooth, wrinkling evenly and deeply when animal contracts or bends. Fully protracted dorsal papillae in life 1–2 cm in length, about 1 mm in width at base, longer on anterior half of body, attenuating sharply in size near anus, distributed irregularly along ambulacra, usually conical, sometimes more elongate and curved when maximally extended, giving animal a spiky appearance ( Fig. 1 View FIGURE 1 D); when not fully extended, papillae appear as rounded bumps or in unrelaxed preserved specimens become fully retracted appearing as spots nearly flush with body wall. Anus terminal, circular, surrounded by five groups of two or three small papillae. Mouth appears subterminal in life, terminal in relaxed and preserved specimens, surrounded by ragged collar of crowded papillae of same colour pattern as, but about half the length of, those on dorsum ( Fig. 1 View FIGURE 1 E). Oral tentacles 27, about 3 mm long, in life pad dark brown, fringed in dark orange, stalk dark brown occasionally with irregular white areas, but becoming uniformly light grey basally and circumorally. Ventral surface of exterior body wall slightly lighter than dorsal surface. Ventral podia, cylindrical, large, rose-coloured fading to white distally, widely spaced and arranged loosely in three longitudinal rows.
Internal anatomy: Calcareous ring, Semperothuria -like (Rowe 1969), 12 mm in diameter of ten nearly confluent elements with wavy posterior margin, radial elements roughly rectangular, about twice as wide as long (4.5 mm), notched anteriorly, interradial elements about half as wide as radial elements, somewhat shorter and pointed anteriorly. Tentacle ampullae about 5.5 mm long, stone canal, irregularly helical, about 8.5 mm long free in coelom, madrepore missing, longitudinal muscles bifid, about 5 mm wide, attached to body wall medially, respiratory trees insert together near the dorsal anterior of cloaca, left tree about 7.2 cm long, right tree about half as long, extending to within plexus of vessels associated with intestine. Polian vesicle one, elongate, about 8 mm long by 4.5 mm wide, gonads numerous, of cylindrical tubules about 1 mm wide, extending 6.5– 7 mm maximally, Cuvierian tubules not present in preserved holotype. Cuvierian tubules never extruded in live specimens.
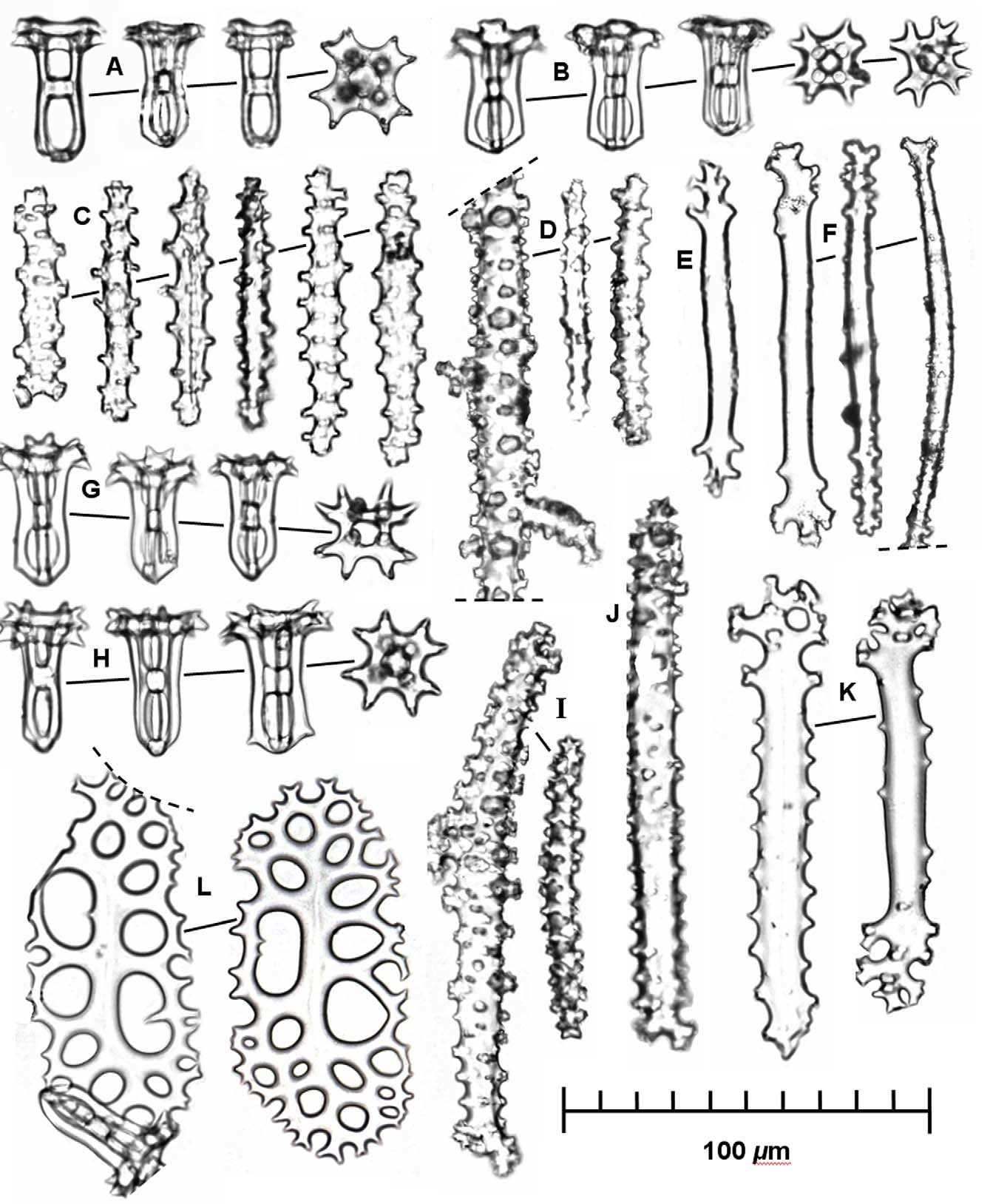
Ossicles: Ossicles quite close in appearance to those of H. flavomaculata , i.e., ossicles in dorsal and ventral body walls also consist primarily of tables and knobby rods ( Fig. 2 View FIGURE 2 ). Table ossicles about 35–45 µm in height with disc absent and instead pillars converging in rounded, occasionally angulate base, sometimes with few small spines; spire high, non-tapering, composed of four pillars slightly constricted medially and joined by single broad crossbeam; terminus of four pairs or occasionally triplets of spines arranged in a "Maltese" cross (sensu Rowe 1969) about 32 µm across with central circular hole about 9 µm in diameter ( Fig. 2 View FIGURE 2 A–B, G–H). Body wall rods usually straight, 50–210 µm, irregular in outline spiny, rarely with perforations at one or both ends in most elongate rods ( Fig. 2 View FIGURE 2 C–D, I–J). Rods in podia and papillae usually curved with scattered spines or knobs and knobby ends, varying in length from about 110–130 µm in length ( Fig. 2 View FIGURE 2 E–F). Tubefeet plates to 120 µm in length, which begin as flattened rods, becoming spiny marginally and perforate terminally, marginal spines bifurcating and anastomosing to form holes, usually circular to oval in outline and arranged in loose rows ( Fig. 2 View FIGURE 2 K–L).
Etymology. The name refers to the many, scattered dorsal papillae that when retracted, especially in an emmersed, hand-held living animal, look like pink spots, from the Latin roseo (the adverbial form of rosa = pink) + macul ā ta (the adjectival form of the feminine macula = spot). The name parallels that of its yellow-spotted sister species, H. (S.) flavomaculata , with which it has long been confused.
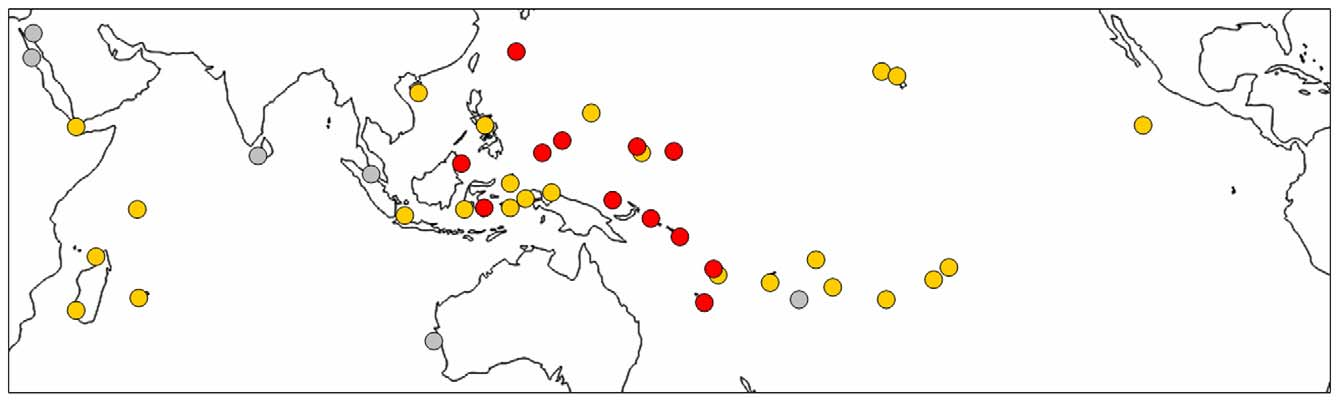
Distribution. Holothuria (S.) roseomaculata n. sp. is known from the westernmost tropical Pacific Ocean ( Fig. 3 View FIGURE 3 ), having been collected or identified from photographs of living animals from southern Okinawa, Japan (F. Michonneau, photo of UF 11021); Sabah, Borneo, Malaysia (D. Lane, photo voucher); the Caroline islands of Chuuk, FSM (Colin & Arneson 1995, as H. flavomaculata ), Palau (this paper), Pohnpei, FSM (this paper) and Yap, FSM (this paper); Bougainville Island (this paper) and Manus Island, PNG (L. Yaman, photo voucher); Marovo Lagoon, Solomon Islands (Buckius et al. 2010, as H. flavomaculata ); Vanuatu (K. Pakoa, photo voucher); and New Caledonia (Féral & Cherbonnier 1986, as H. (S.) flavomaculata ).
The geographic range of H. roseomaculata n. sp. appears to occupy the central portion of the range of its more broadly distributed sister species H. flavomaculata ( Fig. 3 View FIGURE 3 ). As well, collection records from Vanuatu and from Chuuk indicate that the new species is micro-sympatric with the latter at the scales of island, reef and even microhabitat. Some exceptions to island-level sympatry probably occur. For example, despite the new species being widely distributed and locally abundant across much of Micronesia, it appears to be genuinely absent from some islands in this region, such as Guam and Kosrae, two localities subjected to extensive and continuing collecting efforts (Borrero-Pérez et al. in the press; Kerr 1994; Kerr et al. 1992, 1993; Kerr et al. 2008; Kim et al. in the press, Paulay 2003). Intensive collecting for holothuroids in Polynesia (G. Paulay, pers. comm.) has also failed to turn up this species.
There is some difficulty in assessing the distribution of H. roseomaculata n. sp. from literature records because it has long been considered conspecific with H. flavomaculata and papillae colour has sometimes not been reported, e.g., from museum vouchers or published sightings in Egypt, Israel, Sri Lanka, Thailand, and Western Australia. Hence, these records are rendered in grey in Fig. 3 View FIGURE 3 , indicating that at least one of these species is present (in the Indian Ocean, probably only H. flavomaculata ). Further, unpublished government and industry lists of commercially valuable sea cucumbers frequently include a "red snakefish", the trade name uniquely applied to the new species (K. Pakoa, pers. comm.; L. Yaman, pers. comm.). For example, the Fiji Trade and Investment Bureau (FTIB 2012) indicates that sea cucumbers under the name red snakefish are regularly harvested in Fiji for export. Hence, the range of H. roseomaculata n. sp. may extend to the Fijian archipelago, as well.
Monophyly. Until recently, taxonomists have had to rely on relatively robust, but ultimately ad hoc criteria for assessing the species status of a proposed taxon, i.e., via the presence of one or more diagnostic morphological characters. With the advent of phylogenetic analysis, species status is still ascribed informally, usually when exemplars are subtended by a branch deemed of evolutionary length sufficient to suggest speciation. Here, I directly assess the probability of species status cum monophyly of H. roseomaculata n. sp. by using the hypothesis testing approach advocated by Rosenberg (2007; see also Zhu et al. 2011). First, to attain sufficient sample sizes for all tests at a conventional type I error rate of α = 0.05, I re-estimated a phylogeny of clade B from Honey-Escandón et al. (2012) with the authors' 16S sequences (including H. (Halodeima) atra as an outgroup), while including a new 16S sequence of H. flavomaculata (GenBank KC795913 View Materials from USNM E51765 View Materials ). Alignment, phylogenetic estimation and branch-support analysis were carried out using default parametres in Phylogeny.fr (Dereeper et al. 2008). The rooted maximum-likelihood topology (not shown) is identical to that of the original analysis and the new exemplar is recovered in a clade of conspecifics with 100% bootstrap support. Considering this phylogeny and the equations in Rosenberg (2007), the null probability of the a = 3 exemplars of the new species forming the observed monophyletic group amongst the b = 10 other ingroup members was significantly less than expected by chance (P = 0.0076). Further, the new species and H. flavomaculata are confidently reciprocally monophyletic (a = 3, b = 3, P = 0.0200) and provisionally sister, i.e., they jointly form a clade (a = 6, b = 7, P = 0.0004).
Biology. Perhaps because Holothuria (S.) roseomaculata n. sp. can be locally quite abundant, there already exists a small literature on its wider biology under the name H. flavomaculata and its variants that extends to the early 20th century. Yamanouti (1939; Yamanouchi 1956) wrote extensively on the new species' biology as part of comparative studies on aspects of the physiology and behaviour of several species of sea cucumbers abundant on the shallow fringing reef flats of Palau, Micronesia. He found that H. (S.) roseomaculata n. sp. had a gut pH similar to that of other common reef-flat Holothuria and Stichopus spp., but that it consumed the largest proportion of the mud and silt fraction of the sediment. Further, only holothuriids inhabiting clear shallow water, including the new species, ceased feeding when brought into the laboratory. Conversely, the new species fed continuously in situ, unlike most of the other species examined, although the time from ingestion to egestion of H. (S.) roseomaculata n. sp. appeared typical, about five hours. Finally, and unlike most other larger species that Yamanouchi (1956) examined, the new species only rarely played host to the coelomic inquiline pearlfish Carapus (= Encheliophis ) homei (Richardson, 1846) ( Carapidae ).
A few authors have also provided information on the abundance distribution and habitat of the new species. Féral & Cherbonnier (1986) and Conand (1989) report that in New Caledonia the species lives in sheltered waters on soft sediments between 2 and (at least?) 40 m depth and is most active at night, while during the day it is usually retracted in a crevice amongst corals. Conand (1989) also records that the highest densities averaging 76 animals or 45 kg per hectare occurred in several widely separated embayments, all of them with sediments having a noticeable fraction of terrigenous mud and with an abundance of the holothuroids Bohadschia vitiensis (Semper, 1867) and Holothuria (Metriatyla) scabra Jäger, 1833 . Buckius et al. (2010) found the species at a comparable density in a similar habitat in the Solomon Islands, at a single riverine embayment, at 42 animals per hectare on muddy sediments and associated with seagrass to 5 m depth.
Additional observations on the habitat and population density of the new species were made during the course of the present study in the Micronesian islands of Palau, Pohnpei and Yap. The microhabitat and diurnal pattern of activity of the species is unusually varied for a holothuroid. Some animals are fully exposed and epibenthic, feeding diurnally, especially in seagrass meadows on the shallow inner reef at one or two metres depth ( Fig. 1 View FIGURE 1 A). Otherwise, it can be either nocturnally epibenthic or semi-cryptic, i.e., exposing only its anterior from a crevice to feed, often from under live coral ( Fig. 1 View FIGURE 1 D). It is the only Semperothuria to commonly live epibenthically. All other members of the subgenus live cryptically (M. Honey-Escandón, pers. comm.) and of these, only its sister species, H. flavomaculata , also exposes its anterior nocturnally (Kerr et al. 1992).
When Holothuria (S.) roseomaculata n. sp. occurs in shallow reef flats as in seagrass, it often does so in a restricted area and at rather high abundance (Yamanouti 1939; Amesbury et al. 1977; Grosenbaugh 1981; Conand 1989; Buckius et al. 2010; this study). In Yap and Palau, the species occurred only at a few widely separated sites at high abundance. The high densities in Yap appeared associated with the presence of lesions on the dorsal body wall, perhaps opportunistic infections spread by close contact as frequently seen in high-density aquaculture of other coral-reef holothuroids (review in Purcell & Eeckhaut 2005). By contrast, in deeper habitats, as on reef slopes or lagoonal patch reefs, e.g., New Caledonia (Conand 1989), Palau (D. Burdick, pers. comm.), Yap and Pohnpei, FSM (this paper), and Sabah, Malaysia (D. Lane, pers. comm.), the animals are usually seen as isolated individuals and never presented dermal lesions.
Some characteristics of the new species constitute previously unconsidered potential synapomorphies of a more inclusive clade of most Semperothuria spp. (clade B in fig. 1 of Honey-Escandón et al. 2012) or of derived vermiform Holothuria . Many sea cucumbers, including holothuriids, tend to contract greatly and axially when disturbed. However, H. roseomaculata n. sp., like its sister H. flavomaculata , tends to contract only moderately and then fold in half ( Fig. 1 View FIGURE 1 C). This unusual behaviour appears to be a synapomorphy of the clade formed by these two species. Additionally, the bodies of living animals are often invested in a thin layer of mucus that becomes embedded with fine sediment ( Fig. 1 View FIGURE 1 D). This mucoid theca is only seen in several subgenera of presumably derived Holothuria (e.g., Mertensiothuria , Halodeima and Thymiosycia in part). I speculate therefore that this widespread character constitutes a synapomorphy of a large clade of the vermiform Holothuria to which the new Semperothuria sp. belongs.
The dorsal papillae, tubefeet and tentacle colours of H. roseomaculata n. sp. and H. flavomaculata wash out quickly upon immersion in 95% ethanol, consistent with the carotenoid-based pigments common to many echinoderms, including holothuroids (Bandaranayake & Des Rocher 1999). By contrast, the fugitive brown to grey ground colours of their body walls resist dissolution in preservative, though appear to bleach in older bottled specimens from exposure to light, indicating a chemically different class of echinoderm pigments, consistent with melanins (Millott 1953; Kennedy 1979). The distribution of this latter ground pigment in the body wall and tentacles is conserved amongst the two sister species, while the colour of the carotenoid pigment is apomorphic (pink versus yellow, respectively). This raises the more general question: Do the particular hues, or the complete gain and loss, of the carotenoid-like system of pigments usually evolve more quickly or in consistently different ways among holothuroids than those of the latter melanin-based system? If so, do these differences covary with aspects of biology, such as body size, microhabitat, and diurnal pattern of activity?
Conservation status. Like other holothuroids, H. roseomaculata n. sp. has not been assessed for possible inclusion in the IUCN Red List of Threatened Species (IUCN 2012). Moreover, as a previously unrecognised species, one that was considered a color variant of a described form, it has also not yet been included in the draft Red List assessment being prepared for commercially exploited holothuroids (Polidoro et al. 2011). The new species is commercially harvested in small numbers across part of its geographic range, including northern Papua New Guinea (L. Yaman, pers. comm.) and the Solomon Islands (Buckius et al. 2010). It is considered a low-value species (Purcell et al. 2012), the properly prepared dried product "beche de mer" in the Solomon Islands bringing about US $6.00/kg (Buckius et al. 2010). By contrast, beche de mer of the preferred high-value species Actinopyga mauritiana (Quoy & Gaimard, 1833) in these islands can bring US $42.00/kg to local fishers (Buckius et al. 2010). However, as high value species are fished down, there grows increasing collecting pressure on lower valued species, including on H. roseomaculata n. sp. The new species' habit would increase its vulnerability, as most animals lie exposed during the day, sometimes as dense populations in shallow water, making over-harvesting almost certain. For example, Buckius et al. (2010) found that the densities of a shallow-water inshore population of the new species in the Solomon Islands went from an average of 42 individuals per hectare to zero sightings following three months of intense fishing in anticipation of closure of the fishery. Despite this, the conservation status of the new species is not as worrisome as that for intensely harvested species of the most highly prized forms. While shallow-water assemblages of the new species will become quickly fished out, thus vastly reducing their numbers in a fishery, its wide habitat preferences that include nocturnal habits and scattered presence in deeper waters will mean that it is unlikely to be completely extirpated.
Remarks. Previous workers (e.g., Cherbonnier 1980; Féral & Cherbonnier 1986) assigned the new species to Holothuria (S.) flavomaculata primarily because of the two species' similar gross appearance and apparent identical complements of ossicles. In this study, the new species and H. flavomaculata also possessed matching sets of ossicle types in the body wall: primarily, the distinctive " flavomaculata "-like tables, plates (sometimes presenting as flattened spiny rods), and knobby to spiny rods circular in transverse section. Absent a statistical analysis, the table ossicles appear indistinguishable between the two species, as do the plates. However, the knobby rods differ consistently between the species in the geographically broad sampling of examined specimens and among those published in the literature. The rods of H. flavomaculata more often appear strongly fusiform and covered with molariform knobs quite closely packed, i.e., the spaces between the knobs are much less than the widths of the knobs; see Plate XXX, Fig. 26 in Semper (1867) and Plate 27, Fig. 15 in Clark & Rowe (1971). By contrast, the rods of H. roseomaculata n. sp. are more often cylindrical, thinner, and usually possess less developed knobs to short spines that are separated by a distance exceeding the width of the protuberances ( Fig. 2 View FIGURE 2 C–D, I–J; see also Fig. 10B in Cherbonnier 1980). These distinctions admittedly can be unclear in some published illustrations of the rod ossicles, such as in the yellow-papillate H. flavomaculata from Fig. 26C of Cherbonnier (1988) and Fig. 74 of Liao (1997). Fortunately, the occasional subtlety of the ossicle differences does not impede immediate diagnosis of the new species, since the prominent pink dorsal papillae serve as an infallible field character ( Fig. 1 View FIGURE 1 ).
To the memory of the late Chief Charles L. Chieng, Executive Director of the Yap Community Action Program, I dedicate this paper, for his vision and leadership in conserving Micronesia's natural resources. I thank for discussion, distributional information and photo vouchers C. Brunson (UG), D. Burdick (UG), R. Clouse (AMNH), M. Honey-Escandón (UNAM), S. Kim (KIOST), D. Lane (UBD), F. Michonneau (UF), K. Pakoa (SPC), G. Paulay (UF), S. Purcell (SPC), F. Rowe (AM); Y. Samyn (IRSNB), T. Skewes (CSIRO), E. Tardy (SPC), and L. Yaman (National Fisheries Authority, PNG); for loan of specimens, L. Buss (YPM), P. Greenhall (USNM), Eric Lazo- Wasem (YPM), G. Paulay, D. Pawson (USNM) and E. Tardy; for slide preparation and microphotography, C. Brunson, C. Lobban, and A. Miller (all UG); for fieldwork in Yap, M. Hoffman (USF), S. Kim, and K. Netchy (USF); for logistic support, the Yap State Government, FSM, especially M. Gaan, M. Hasurmai, D. Mailing and A. Tafiliechig; and for permission to collect, the Yapese Traditional Council of Chiefs. This work was funded by the Marine Resources Pacific Consortium ( U.S. Department of the Interior Office of Insular Affairs) to R. Richmond (UH), NSF PEET program grant 0 529724 to G. Paulay and AMK, and NSF AToL program grant 1036229 to AMK. This is a contribution of the Marine Laboratory, University of Guam.
References
Amesbury, S.S., Tsuda, R.T., Randall, R.H. & Birkeland, C.E. (1977) Marine biological survey of the proposed dock site at Colonia, Yap. University of Guam Marine Laboratory Technical Report, 35, 1 –22.
Bandaranayake, W.M. & Des Rocher, A. (1999) Role of secondary metabolites and pigments in the epidermal tissues, ripe ovaries, viscera, gut contents and diet of the sea cucumber Holothuria atra. Marine Biology, 133, 163–169.
Borrero-Pérez, G.H., Honey-Escandón, M., Kamarudin, K.R., Kerr, A.M., Kim, S., Meñez, M.A., Michonneau, F., Miller, A., Ochoa, J.A., Olavides, R.D., Paulay, G., Samyn, Y., Setyastuti, A., Solís-Marín, F., Starmer, J. & VandenSpiegel, D. (2013) The littoral sea cucumber (Echinodermata: Holothuroidea) fauna of Guam re-assessed — a diversity curve that still does not asymptote. Cahiers de Biologie Marine. [in press]
Buckius, C., Albert, S., Tibbetts, I. & Udy, J. (2010) Effect of diel activity patterns and harvesting pressure on the diversity and biomass of sea cucumbers in Marovo Lagoon, Solomon Islands. Environmental Management, 45, 963–973.
Cherbonnier, G. (1955) Holothuries récoltées en Océanie française par G. Ranson en 1952. 2e note. Bulletin Muséum National Histoire Naturelle Paris, 2 série, 27, 77–82, 2 figs.
Cherbonnier, G. (1980) Holothuries de Nouvelle-Calédonie. Bulletin Muséum National Histoire Naturelle Paris, 4 série, 2A 3, 615–667.
Clarke, A.M. & Rowe, F.W.E. (1971) Monograph of Shallow-Water Indo-West Pacific Echinoderms. Trustees of the British Museum (Natural History), London, 277 pp.
Colin, P.L. & Arneson, C. (1995) Tropical Pacific Invertebrates. Coral Reef Press, Beverly Hills CA, 296 pp.
Conand, C. (1989) Les Holothuries aspidochirotes du Lagon de Nouvelle-Calédonie: Biologie, Écologie et Exploitation. Collection Etudes et Thèse. Éditions de l’ORSTOM, Noumea, New Caledonia, 393 pp.
Deichmann, E. (1958) The Holothuroidea collected by the VELERO III and IV during the years 1932 to 1954. Part II. Apsidochirota. Allan Hancock Pacific Expeditions, 11 (2), 239–349, 9 pls.
Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M. & Gascuel O. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research, 36, W465–W 469.
Féral, J.-P. & Cherbonnier, G. (1986) Les holothurides. In: Guille, A., Laboute, P. & Menou, J.L. (Eds.) Guide des Étoiles de Mer, Oursins et autres Échinodermes du Lagon de Nouvelle-Calédonie. Faune Tropicale 25. Éditions de l’ORSTOM, Noumea, New Caledonia, pp. 57–107.
Friedman, K., Ropeti, E. & Tafileichig, A. (2008) Development of a management plan for Yap's sea cucumber fishery. SPC Beche-de-mer Information Bulletin, 28, 7 –13.
FTIB ( Fiji Trade and Investment Bureau) (2012) Investment Fiji: Sea cucumber/Beach-de-mer [sic]. http://www.investmentfiji.org.fj / pages.cfm/for-investors/industry-project-profiles-1/sea-cucumber-beach-de-mer.html (Accessed 5 June 2012)
Grosenbaugh, D.A. (1981) Qualitative assessment of the asteroids, echinoids and holothurians in Yap lagoon. Atoll Research Bulletin, 255, 49 –54.
Honey-Escandón, M., Laguarda-Figueras, A. & Solís-Marín F.A. (2012) Molecular phylogeny of the subgenus Holothuria (Selenkothuria) Deichmann, 1958 (Holothuroidea: Aspidochirotida). Zoological Journal of the Linnean Society, 165, 109 – 120.
ICZN (International Commission on Zoological Nomenclature) (1924) Opinion 80. Suspension of rules in the case of Holothuria and Physalia. Smithsonian Miscellaneous Collections, 73, 17–18.
IUCN (International Union for the Conservation of Nature) (2012) The IUCN Red List of Threatened Species. Version 2012.2. http://www.iucnredlist.org. (Accessed 1 November 2012)
Kennedy, G. Y. (1979) Pigments of marine invertebrates. Advances in Marine Biology, 16, 309–356.
Kerr, A.M. (1994) The shallow-water holothuroids (Echinodermata) of Kosrae, Eastern Caroline Islands. Pacific Science, 48, 161–174.
Kerr, A.M., Kim, S.W., & Michonneau, F. (2008) The shallow-water echinoderms of Kosrae. University of Guam Marine Laboratory Technical Report, 123, 1 –27.
Kerr, A.M., Netchy, K.N. & Hoffman, S.M. (2007) The shallow-water echinoderms of Yap. University of Guam Marine Laboratory Technical Report, 121, 1 –34.
Kerr, A.M., Norris, D.R., Schupp, P.J., Meyer, K.D., Pitlik, T.J., Hopper, D.R., Chamberlain J.D. & Meyer, L.S. (1992) Range extensions of echinoderms (Asteroidea, Echinoidea and Holothuroidea). Micronesica, 25, 201–216.
Kerr, A.M., Stoffell, E.M. & Yoon, R.L. (1993) Abundance distribution of holothuroids (Echinodermata: Holothuroidea) on a windward and leeward fringing coral reef, Guam, Mariana Islands. Bulletin of Marine Science, 52, 780–791.
Kim, S.W. (2010) Molecular Systematics of the Tropical Sea Cucumbers Bohadschia (Holothuriidae: Holothuroidea). Master of Science Thesis, University of Guam, Mangilao, Guam, U.S.A., 80 pp.
Kim, S.W., Kerr, A.M. and Paulay, G. (2013) Color, confusion and crossing: resolution of species problems in Bohadschia (Echinodermata: Holothuroidea). Zoological Journal of the Linnean Society. [in press]
Kim, S.W., Miller, A.J., Brunson, C., Netchy, K., Clouse, R., Janies, D., Tardy, E. & Kerr, A.M. (2013) Shallow-water holothuroids (Echinodermata) of Yap, Federated States of Micronesia. Pacific Science. [in press]
Liao, Y. (1997) Fauna Sinica. Phylum Echinodermata. Class Holothuroidea. Science Press, Beijing, 334 pp. [In Chinese]
Ludwig, H.L. (1888) Die von Dr. J. Brock im Indischen Archipel gesammelten Holothurien. Zoologische Jahrbücher.
Abteilung für Systematik, Geographie und Biologie der Tiere, 3, 805–820.
Massin, C. (1999) Reef-dwelling Holothuroidea (Echinodermata) of the Spermonde Archipelago (South-west Sulawesi, Indonesia). Zoologische Verhandelingen, 329, 1 –144, 114 figs.
Millott, N. (1953) Observations in the skin pigment and amoebocytes, and the occurrence of phenolases in the coelomic fluid of Holothuria Forskali Delle Chiaje. Journal of the Marine Biological Association of the United Kingdom, 31, 529–539.
O'Loughlin, P.M., Paulay, G., VandenSpiegel, D. & Samyn, Y. (2007) New Holothuria species from Australia (Echinodermata: Holothuroidea: Holothuriidae), with comments on the origin of deep and cool holothuriids. Memoirs of the Museum Victoria, 64, 35 –52.
Paulay, G. (2003) The Asteroidea, Echinoidea, and Holothuroidea (Echinodermata) of the Mariana Islands. Micronesica, 35– 36, 563–583.
Polidoro, B., Tognelli, M., Harwell, H., Elfes, C., Cepeda, A.A., González-Maya, J.F., Zárrate-Charry, D.A., Alvarado, J.J., Benavides, M., Conand, C., Ortiz, E.P., Gamboa, R., Hamel, J.F., Mercier, A., Purcell, S. & Toral-Granda, V. (2011) IUCN Red List workshop for sea cucumbers. SPC Beche-de-mer Information Bulletin, 31, 65.
Purcell, S. & Eeckhaut, I. (2005) An external check for disease and health of hatchery-produced sea cucumbers. SPC Beche-demer Information Bulletin, 22, 34–38.
Purcell, S.W., Samyn, Y. & Conand, C. (2012) Commercially important sea cucumbers of the world. FAO Species Catalogue for Fishery Purposes, 6, 1–150.
Rosenberg, N.A. (2007) Statistical tests for taxonomic distinctiveness from observations of monophyly. Evolution, 61, 317– 323.
Rowe, F.W.E. (1969) A review of the family Holothuriidae. Bulletin British Museum Natural History, Zoology, 18 (4), 117–170.
Selenka, E. (1868) Beiträge zur Anatomie und Systematik der Holothurien. Zeitschrift Wissenschaftliche Zoologie, 17, 291– 374., pls. 17–20.
Semper, C. (1868) Reisen im Archipel der Philippinen. Zweiter Theil. Wissenschaftliche Resultate. Erster Band. Holothurien. Hefte iv–v. Wilhelm Engelmann, Leipzig, unpaginated. pp. 1868–1916.
Sluiter, C.P. (1895) Die Holothurien Sammlung des Museum im Amsterdam. Bijdragen Dierkunde, 17, 75–82.
Tardy, E. & Pakoa, K. (2009) The status of sea cucumbers in Yap State, Federated States of Micronesia. Secretariat of the Pacific Community, Noumea, 68 pp.
Théel, H. (1886) Report on the Holothurioidea dredged by the H.M.S. Challenger during the years 1873–1876. Part II. Scientific Results of the H.M.S Challenger 1873–1876. Zoology IV(34), 1–290, 16 pls.
Yamanouchi, T. (1956) The daily activity rhythms of the holothurians in the coral reef of Palau Islands. Publications of the Seto Marine Biological Laboratory, 5, 347–362.
Yamanouti, T. (1939) Ecological and physiological studies on the holothurians in the coral reef of Palao Islands. Palao Tropical Biology Station Studies, 4, 603–636.
Zhu, S., Degnan, J.H. & Steel, M. (2011) Clades, clans, and reciprocal monophyly under neutral evolutionary models. Theoretical Population Biology, 79, 220–227.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.