Sinantherina socialis subsp. function
|
publication ID |
https://doi.org/ 10.1016/j.jcz.2023.03.001 |
|
persistent identifier |
https://treatment.plazi.org/id/2833B20C-2753-FFED-FFB4-F9C9A4EE26AD |
|
treatment provided by |
Felipe |
|
scientific name |
Sinantherina socialis subsp. function |
| status |
|
4.2. Do the warts of S. socialis function as exocrine glands?
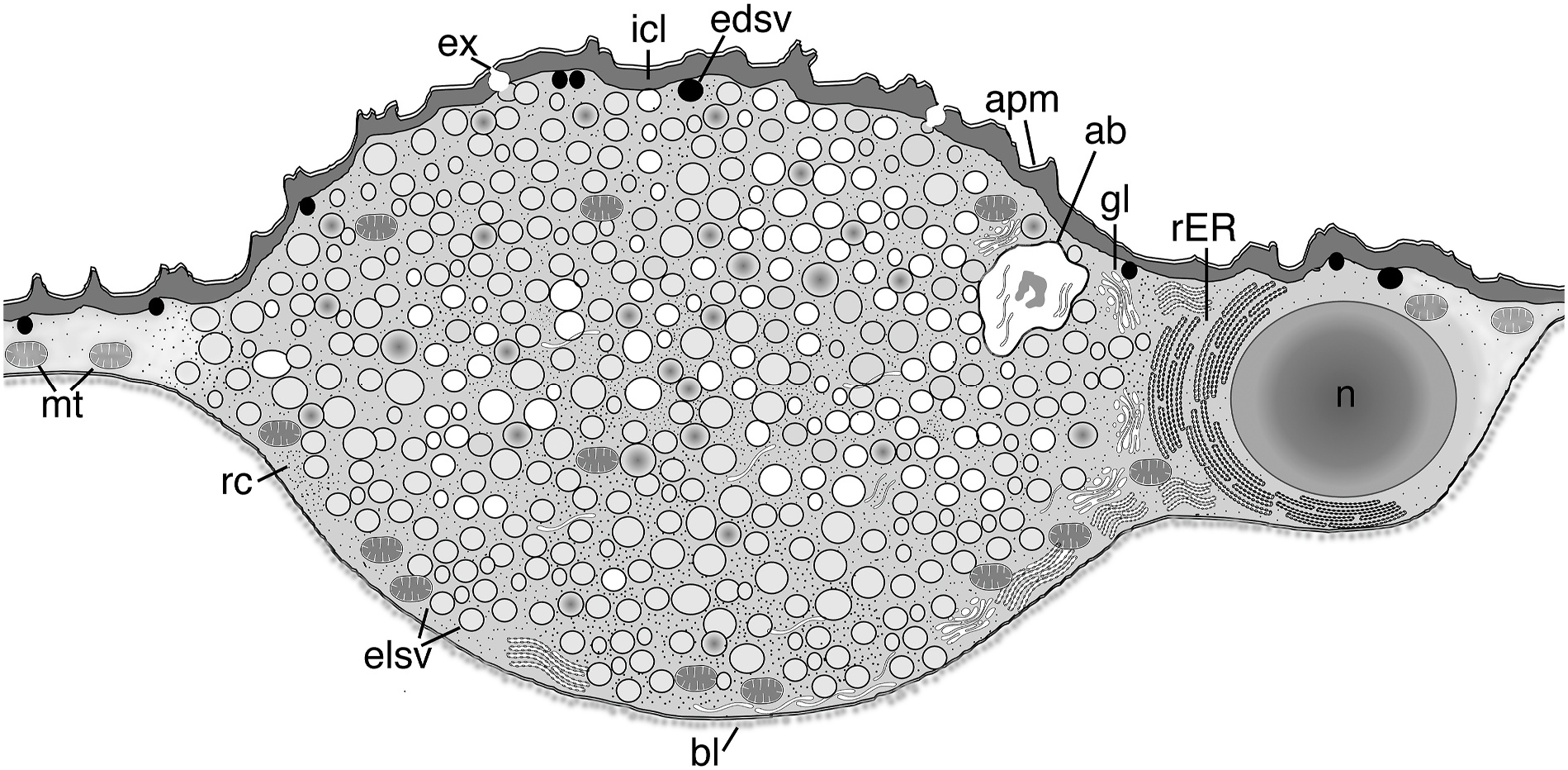
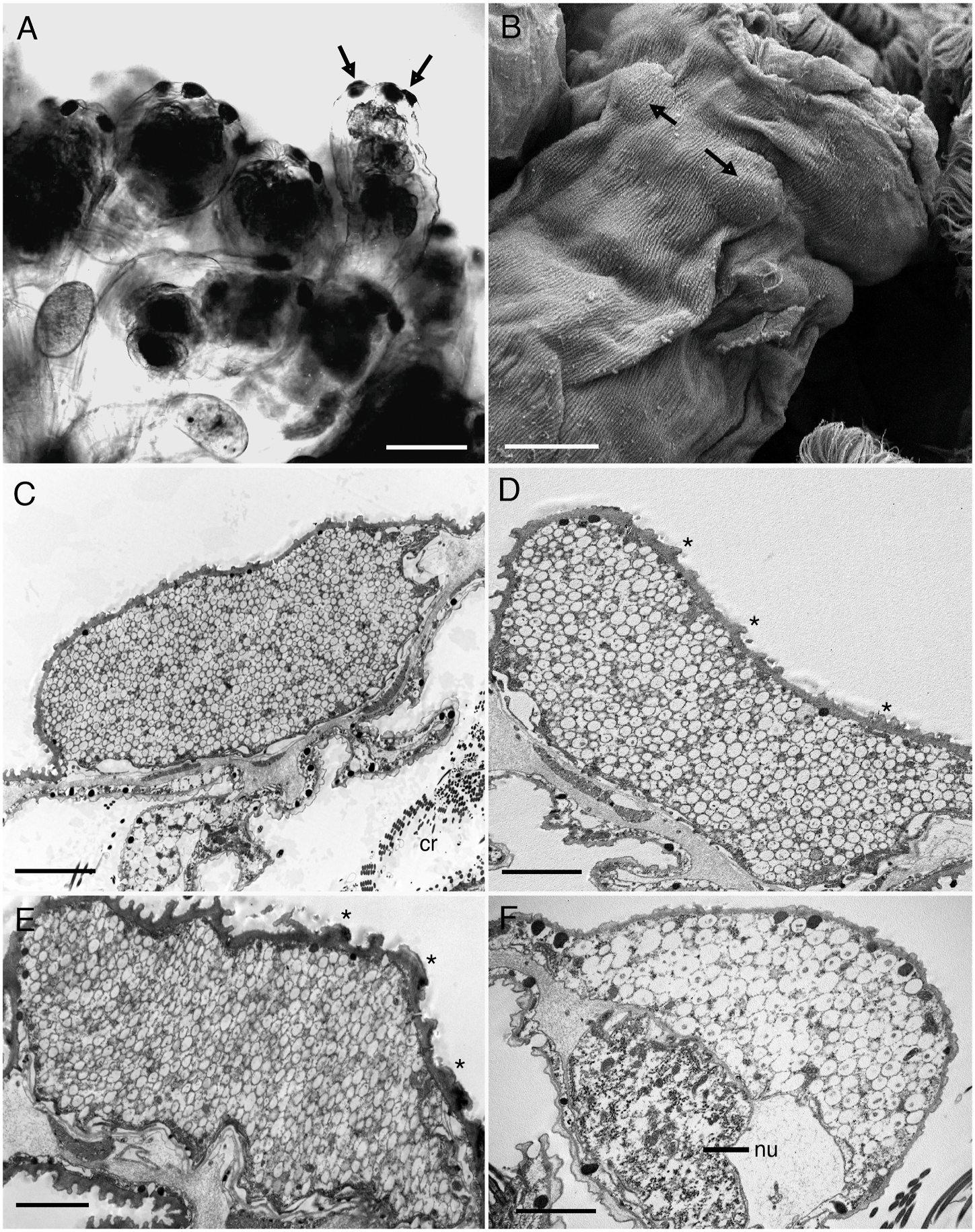
Rotifer glands have not been well studied, but recent studies reveal that most glands, except for pedal glands that function in attachment, are syncytial ( Yang and Hochberg, 2018; Yang et al., 2021). Rotifer glands appear to be restricted to specific areas within the syncytial integument, and in these regions are easily recognized by possessing a high abundance of synthetic machinery (e.g., ER, Golgi) and a high density of secretory vesicles or granules. For example, species of Limnias have trunk glands that are merely localized regions of the integument containing abundant organelles and secretions relative to the rest of the integument. These glands undergo regulative secretion, meaning they appear to store secretory vesicles for a period before exocytosis releases the gland contents through the apical plasma membrane and outside their bodies where a protective tube is built ( Yang and Hochberg, 2018). In S. socialis , the warts are clearly glandular as they contain abundant organelles and numerous secretory vesicles within a defined region of the integument, similar to Limnias . Transmission electron microscopy reveals the warts of S. socialis to be independent, localized swellings of the integument without intracellular plasma membranes, i.e., they are part of the integumentary syncytium ( Fig. 7 View Fig ). There is no obvious connection between the four warts other than through the continuum of the syncytial integument. Each wart is swollen relative to the integument around it: the swelling appears spindle-shaped with the center of the spindle filled with ribosome-rich cytoplasm, mitochondria, rER, and hundreds of membrane-bound secretion vesicles. A nucleus is usually present off center. Most secretion vesicles are oval to round, have electron-lucent cores, and range in size from 149 to 201 nm diameter (x = 175.5 nm ± 17.7 nm). Vesicles with electron-dense cores were occasionally present and measured on average 292 ± 44.6 nm; these vesicles were not unique to the swollen syncytium but were observed scattered across the integument. All wart-contained vesicles appeared destined for transport across the intracytoplasmic lamina (ICL), an intracellular, cuticle-like region that is positioned just below the plasma membrane of the integument. These vesicles appear to fuse individually with the ICL and release their contents en masse; this differs from the normal form of exocrine secretion in the rotifer integument where vesicles fuse with the plasma membrane one at a time (or at least not all at once) ( Koehler, 1965; 1966; Yang et al., 2021). Vesicle fusion is probably followed by a recycling of the bounding membranes, which is characteristic of glands that undergo regulative secretion ( De Camilli and Jahn, 1990; K¨ogel and Gerdes, 2009). Hence, there is no permanent pore to these glandular warts.
The behavior that probably leads to the synchronized expulsion of gland contents is longitudinal body contraction, which is observed when predators touch colony members ( Felix et al., 1995). The glands are not enclosed in muscles nor directly innervated, which supports this hypothesis. However, this would also mean that each time a member contracts, regardless of the reason, secretions are exuded. Such a behavior would require continuous production of secretory vesicles, and so different members of a colony may therefore have different abundances of vesicles in their warts at any one time. We did not compare vesicle amounts between colony members, but observations suggest vesicle numbers appeared similar within individuals, i.e., all warts were either full or empty in a single specimen. Curiously, we have never observed increased evidence of exocytosis in contracted (preserved) specimens, so there must be other factors that lead to simultaneous expulsion of the secretion products. Interestingly, we did process a single contracted specimen from a separate colony that had "empty" warts. These warts were of a similar size and shape to warts filled with secretions in other individuals, but the cytoplasm lacked the secretion vesicles present in other specimens (compare Figs. 4 View Fig and 6 View Fig ), and the cytoplasm was virtually devoid of free ribosomes. Instead, it contained an abundance of vesicle-shaped bodies, some with electron-lucent cores, and others with disrupted membranes that were peeling inward to the core or externally unraveling (see Fig. 6B View Fig ). Whether these bodies represent empty secretion vesicle membranes is unknown, but if they are, then their appearance suggests that the secretion vesicles have multiple membranes, which to our knowledge would be unusual. The specimen with "empty" warts did not have any obviously secreted contents on the outside of the integument, nor did we observe any other specimens (live specimens with light microscopy or preserved specimens with scanning electron microscopy) that showed evidence of exuded secretory material. Of course, clear gland contents would be difficult to detect in situ and the secretions may rinse off or dissolve during preparation for EM.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.