Axis axis ( Erxleben, 1777 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/seab006 |
|
publication LSID |
lsid:zoobank.org:pub:2BFE65E7-0F17-4305-A9CB-59C14BD7830D |
|
persistent identifier |
https://treatment.plazi.org/id/2963879A-FE1A-925D-FECA-FBAE1F76FBA7 |
|
treatment provided by |
Felipe |
|
scientific name |
Axis axis ( Erxleben, 1777 ) |
| status |
|
Axis axis ( Erxleben, 1777) View in CoL
Chital
[ Cervus View in CoL ] Axis Erxleben, 1777:312 View in CoL . Type locality “habitat ad ripas Gangis, vulgaris; in Iaua [sic], Ceylona [= common habitat on the banks of the Ganges; on “Illa Iana,” Ceylon (= Sri Lanka)].”
C [ervus]. Axis maculatus Kerr, 1792:300 . Type locality “banks of the Ganges and the island of Ceylon (= Sri Lanka).”
Cervus axis: Hamilton-Smith, 1827a:117 View in CoL . Name combination.
Cervus axis var. ceylonensis J. B. Fischer, 1829:619 (page numbered as 419 due to error). Type locality “ In campis prope Surpat Indiae orinetalis et in sylvis densis Indiae orientalis insularumque Ceylon, Javae et Sumatrae. ”
Cervus axis var. indicus J. B. Fischer, 1829:619 (page numbered as 419 due to error). Type locality “ In campis prope Surpat Indiae orinetalis et in sylvis densis Indiae orientalis insularumque Ceylon, Javae et Sumatrae. ”
Cervus nudipalpebra Ogilby, 1831:136 . Type locality “Banks of the Ganges.”
Axis maculatus: Jardine, 1835 :un-numbered page (in Table of Contents). Name combination.
Axis aculatus Jardine, 1835:167 . Incorrect subsequent spelling of Cervus axis maculatus Kerr, 1792 .
Cervus (Axis) major Hodgson, 1841:914 . Type locality “ Nepal.”
Cervus (Axis) minor Hodgson, 1841:914 . Type locality “ Nepal.”
Axis maculata: Gray, 1843:178 . Name combination and unjustified emendation of Cervus axis maculatus Kerr, 1792 .
C [ervus (Hippelaphi)]. axis: Sundevall, 1846:177 View in CoL , 180. Name combination.
C [ervus]. axi Sundevall, 1846:180. Incorrect subsequent spelling of Cervus axis Erxleben, 1777 .
Axis maculatus: Jerdon, 1867:260 . Correction of gender agreement.
Hyelaphus maculatus: Fitzinger, 1874:259 . Name combination.
Axis maculata ceylonensis: Fitzinger, 1874:269 . Name combination.
Axis nudipalpebra: Fitzinger, 1874:270 . Name combination.
Cervus (Rusa) axis zeylanicus Lydekker, 1905:947 . Type locality “ Ceylon (= Sri Lanka).”
Axis axis: R. I. Pocock, 1923:184 View in CoL . First use of current name combination.
Chital cervus axis: A. S. Griffith, 1928:206 View in CoL . Name combination.
Cervus axis maculates Groves, 2003:351 . Incorrect subsequent spelling of Cervus axis maculatus Kerr, 1792:300 .
CONTEXT AND CONTENT. Context as for genus. Axis axis View in CoL is monotypic.
NOMENCLATURAL NOTES. Although Hamilton-Smith (1827b) is usually considered the authority of the genus Axis (e.g., Grubb 2005; Groves and Grubb 2011), Kretzoi and Kretzoi (2000a, 2000b) refer to “de Blainville 1816 -?-” as the authority with the annotation “fide [trust in] Gray 1825.” Gray (1825) predates Hamilton-Smith (1827b), and used Axis as one of eight genera in his tribe Cervina and presented it as “ Axis, Blainv. ” Uncertain of the true publication date of de Blainville in which Axis was presented, Kretzoi and Kretzoi (2000a, 2000b) trusted Gray’s reference to an earlier publication and guessed that the year 1816 was correct. The 1816 publication by de Blainville represents his earliest classification of “ruminans” but does not include the name Axis . Later, de Blainville (1822) published a detailed classification of Cervus , in which he grouped 10 Asian species under the vernacular name “ Axis .” He did present one of those species as “L’Axis unicolor, A. unicolor ,” possibly the first use of “ A [xis]” as a genus or a lapsus. Despite de Blainville’s (1822) usage of “ Axis ” as a vernacular name and potential for “ A. unicolor ” to be a reference to “ Axis ” as a genus, his intent is unclear. Thus, the most straightforward first use of Axis as a genus is Gray (1825), and therefore he is considered the authority.
DIAGNOSIS
The subfamily Cervinae consists of nine genera found almost entirely in Eurasia; seven of the nine genera in Cervinae are currently in the tribe Cervini : Axis , Cervus , Dama , Elaphurus , Przewalskium , Rucervus , and Rusa (Groves and Grubb 2011) . Among the five species of Axis ( axis , annamiticus [Indochina hog deer], calamianensis [Calamian deer], kuhlii [Bawean deer], and porcinus [Indian hog deer]), distribution differentiates Axis axis from all congeneric species. In areas where A. axis has been introduced, adults can be differentiated from co-occurring species, excluding Dama dama (common fallow deer), by their spotted coat. Where A. axis co-occurs with common fallow deer, adult A. axis may be differentiated from the common fallow deer by its more reddish coat, stronger dorsal stripe, and palmate antlers in the male.
GENERAL CHARACTERS
Axis axis is a medium-sized, heavily spotted deer standing 0.6–1.0 m at the shoulder with an average overall body length of 1.5 m ( Walker 1964), and is the third largest ungulate on the Indian subcontinent (Karanth and Sunquist 1992; Khan et al. 1995) with Boselaphus tragocamelus (nilgai— Leslie 2008) and Rusa unicolor (sambar— Leslie 2011) being the largest and second largest ungulates, respectively, on the Indian subcontinent. A. axis has white spots, often aligning in longitudinal rows, on its flanks and back over a reddish coat, with a dark brown-to-black stripe running from the base of the neck to the base of the tail; inner legs, belly, throat patch, and underside of the tail are white ( Walker 1964; Ables et al. 1977). Males grow large, simple antlers, typically with three tines per side; each triad has a relatively short, forward-curved brow tine, an elongated frontal tine, and the long tip of the main shaft forms the third tine ( Fig. 1 View Fig ; Grubb 1990).
DISTRIBUTION
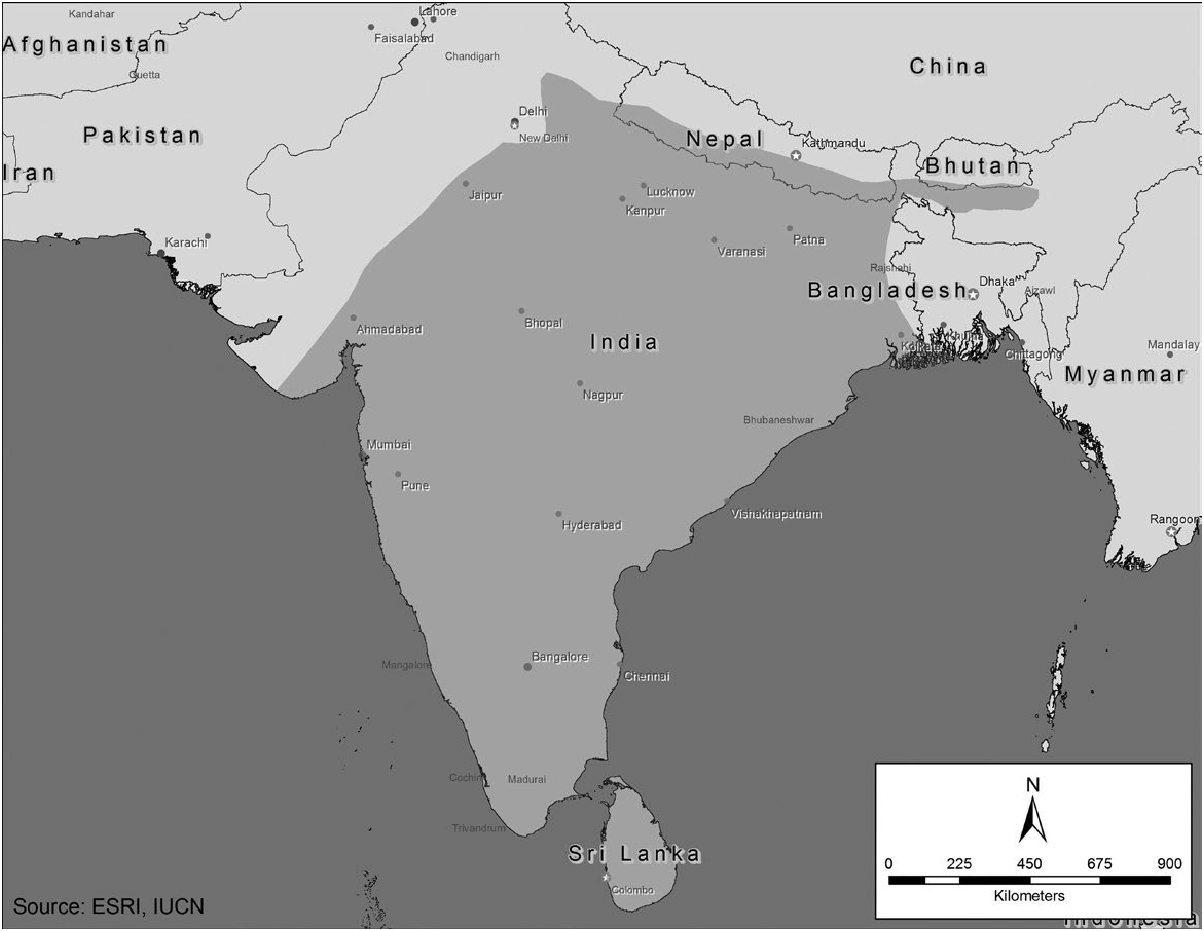
Axis axis is native to south Asia, occurring between 8 and 30°N in Bhutan, Bangladesh, India, Nepal, Pakistan, and Sri Lanka ( Prater 1934; Schaller 1967; Duckworth et al. 2015), with the Himalayan foothills forming the northern boundary of distribution ( Walker 1975). Free-living and captive populations have been established in Australia, the continental United States (Graf and Nichols 1966; Ables et al. 1977; Brooks 2006), the Hawaiian Islands ( Tomich 1986), and South America ( Petrides 1975; Crespo 1982; Lever 1985; Navas 1987; Carpinetti and Merino 1999; Sponchiado et al. 2011), and Europe ( Grubb 2005; Fig. 2 View Fig ).
FOSSIL RECORD
Artiodactyls first appear in the fossil record in the Eocene epoch with widespread occurrences throughout North American and Eurasian deposits 46 million years ago ( Rose 1982, 1996). Tectonic uplifts forming the Himalayas and Alps during the Oligocene epoch lead to significant diversification of deer-like forms with the emergence of Cervids during the late Oligocene; the Cervinae subfamily dates from the central Asian fossil records 7–9 million years ago, during the late Miocene ( Bubenik 1990; Petronio et al. 2007).
The first recognizable Cervini forms are from early and middle Pliocene deposits of central and western Asia (Teilhard de Chardin and Trassaert 1937; Flynn et al. 1991; Di Stefano and Petronio 2002). Current evolutionary evidence suggests that Axis diverged from Cervus about 6 million years ago with an additional divergence between Axis and Rucervus about 5 million years ago ( Pitra et al. 2004), representing the earliest divergence in tribe Cervini ( Gilbert et al. 2006) .
Axis shansius is the first recorded fossil specimen of Axis , identified from the Yushé Basin, southern Shansi, China (Teilhard de Chardin and Trassaert 1937), and is the earliest fossil specimen recorded in the Axis lineage (Di Stefano and Petronio 2002). A. shansius has been located throughout Eurasia including China (Teilhard de Chardin and Trassaert 1937), Russia ( Vislobokova et al. 1995), and the Guide Basin, Tibet ( Pares et al. 2003). Within the Plio-Pleistocene deposits, species of Axis and Rusa were characterized in zone II of the Yushé series and classified into the Indo-Malayan group (Teilhard de Chardin and Trassaert 1937; Otsuka 1969). Axis fossils are characteristic of the Nihowan and Kuchinotsu fauna assemblages of Japan ( Otsuka 1969), with A. japonicus ( Otsuka 1967) having a less intimate relationship to the Chinese Axis species described by Teilhard de Chardin and Trassaert (1937). A. japonicus is thought to be more closely related to the Trinil and Djetis faunas of Java and might be an ancestral form to A. javanicus and A. lydekkeri ( Otsuka 1969) .
Fossil remains from the Khok Sung site, Thailand, are the first reported for A. axis in Southeast Asia ( Suraprasit et al. 2016). Most A. axis fossils are known from the upper Pleistocene to Holocene deposits in India including the Narmanda Valley (Badam and Sankhyan 2009), Son Valley ( Badam 2002), Manjra Valley ( Badam et al. 1984), the Kurnool cave-complex in the Nandyal Basin ( Chuahan 2008), and the Tarafeni Valley in West Bengal ( Dassarma et al. 1982; Basak et al. 1998).
FORM AND FUNCTION
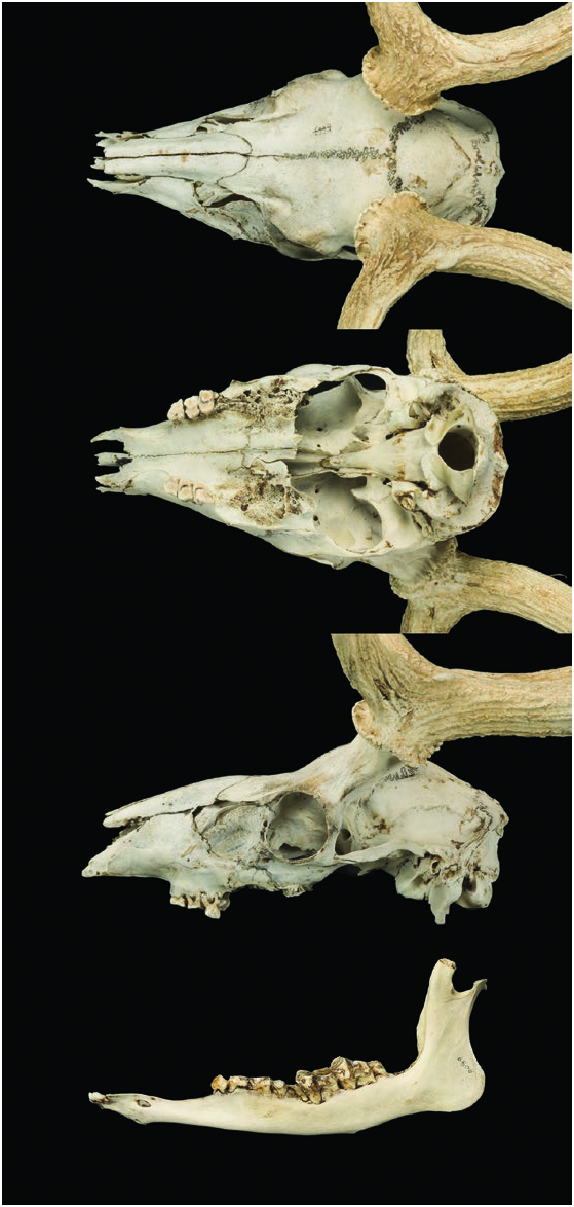
Form. —The skull of Axis axis is composed of two major regions: the facial and cranial parts ( Fig. 3 View Fig ). The shape is dolichocephalic with the facial portion being longer than the cranial portion, forming a roughly triangular shape ( Ramswarup et al. 2014). The maximum skull length is 25.5 cm, and maximum skull width is 10.2 cm. The dental formula for A. axis is: i 0/3, c 0/1, p 3/3. m 3/3, total 32; canines are incisiform as in all Artiodactyla ( Ungar 2010) .
The pelvic girdle (os-coxae) consists of two parts meeting at the pelvic symphysis in the midline and is described as a flat irregular bone ( Yadav et al. 2012). A detailed description of the pelvic bones is provided by Yadav et al. (2012).
Heart morphology of A. axis resembles that of an adult goat, having two surfaces: a broad base and narrow apex (n = 4— Gupta et al. 2015). Mean heart weight (± SE) was 353.75 ± 2.39 g with a length from base to apex of 13.51 ± 0.04 cm ( Gupta et al. 2015). Diameter was 9.80 ± 0.04 cm (sagittal) and 7.58 ± 0.04 cm (transverse); circumference was 24.12 ± 0.42 cm (coronary groove), 19.00 ± 0.40 cm (middle of heart), and 10.65 ± 0.06 cm (junction of left and right longitudinal grooves— Gupta et al. 2015).
The tongue of A. axis is fairly elongated and terminates in an oval tip (Erdoğan and Pérez 2014). Mean (± SE) tongue length from root to apex is 123.89 ± 3.10 mm, and it is uniformly wide along its length: 26.04 ± 0.57 mm (apex), 28.41 ± 1.08 mm (body), 35.23 ± 1.39 mm (torus), and 32.43 ± 0.46 mm (radix). The greatest thickness is 34.24 ± 0.87 mm and found at the torus. Three mechanical papillae (filiform, lenticular, and conical) and two gustatory papillae (circumvallate and fungiform) are found on the dorsal surface of the tongue (n = 5—Erdoğan and Pérez 2014).
Mean (± SE) solar surface (n = 4) of the fore hoof was 6.56 ± 0.024 cm long laterally, with maximum solar surface width of 2.09 ± 0.013 cm; mean (± SE) maximum lateral surface was 3.28 ± 0.014 cm; and mean (± SE) maximum coronet was 0.58 ± 0.014 cm ( Tomar et al. 2011). Mean (± SE) interdigital border was 3.48 ± 0.014 cm long, and mean (± SE) posterior hoof surface was 1.39 ± 0.013 cm. Mean (± SE) hoof angle was 45.0 ± 0.41°. Mean (± SE) solar surface of the hind hooves was 5.79 ± 0.013 cm long laterally, with maximum solar surface width of 1.73 ± 0.014 cm; mean (± SE) maximum lateral surface was 3.40 ± 0.02 cm; and mean (± SE) maximum coronet was 1.23 ± 0.014 cm. Mean (± SE) interdigital border was 3.41 ± 0.013 cm long, and mean (± SE) posterior hoof surface was 1.89 ± 0.013 cm ( Tomar et al. 2011).
Function.— Mean (± SE) rectal temperature was 39.2 ± 0.4°C, mean (± SE) heart rate was 75.5 ± 6.5 beats/min, and mean (± SE) respiratory rate was 62.1 ± 4.2 breaths/min, based on eight chemically immobilized captive A. axis ( Arnemo et al. 1993) . Basic hematology of captive Axis axis was: hemoglobin, 109.0– 171.2 g /l; mean corpuscular hemoglobin concentration, 327–368 g /l; red blood cells 10.22–14.27 × 1012 /l; hematocrit (ratio of red blood cell volume to total blood volume), 0.34–0.47 l/l; white blood cell count, 3.92–8.40 × 109 /l; and mean corpuscular volume, 10.94–12.47 pg ( Chapple et al. 1991). Serum constituent ranges were: protein, 56.3–70.2 g /l; albumin, 27.5–38.3 g /l; alkaline phosphatase, 118–2,493 U/l; creatine kinase, 294–7,400 U/l; calcium, 2.3–2.7 mmol/l; and phosphorus, 2.2–2.7 mmol/l (n = 37— Chapple et al. 1991).
Testis volume (± SE) was greater in hard-antlered males (118.8 ± 4.6 cm 3) than velvet-antlered males (74.6 ± 4.4 cm 3 — Umapathy et al. 2007). Serum testosterone (± SE) was also greater in hard-antlered males (1.2 ± 0.1 ng /ml) than velvetantlered males (0.6 ± 0.1 ng /ml— Umapathy et al. 2007). Hardantlered males additionally had higher semen volume (± SE; 4.1 ± 9.6 ml) and sperm concentration (338.3 ± 24.9 × 106 ml−1) compared to velvet-antlered males semen volume (3.2 ± 0.2 ml) and sperm concentration (57.3 ± 12.4 × 106 ml−1 — Umapathy et al. 2007). Morphologically abnormal sperm were more prevalent (± SE) in velvet-antlered males (43.8 ± 4.1%) than hardantlered males (20.9 ± 1.9%— Umapathy et al. 2007). Motile sperm percentages (± SE) were more than double in the ejaculate of hard-antlered males (66.5 ± 1.5%) than velvet-antlered males (29.9 ± 4.9%— Umapathy et al. 2007). In a study of captive A. axis , motile spermatozoa were collected during all phases of the antler development cycle (Loudon and Curlewis 1988).
As a tropical or subtropical species the antler cycle in A. axis does not appear to be correlated to photoperiod as it is with temperate cervid species ( Grubb 1990). Increases in plasma testosterone levels have been positively correlated with promotion of antler development, and decreasing plasma testosterone levels begin the mineralization process and lead to the ultimate shedding of antlers at completion of the antler growth cycle (Goss and Rosen 1973).
ONTOGENY AND REPRODUCTION
Ontogeny.— A sigmoid growth curve for weight characterizes fetal development of Axis axis ( Chapple 1989) . Conversely, increases in fetal length are approximately linear at about 13 mm /week. Parturition can occur throughout the year, with birth peaks varying widely by geographic location ( Ables et al. 1977; Dinerstein 1980; Mylrea et al. 1999; Ahrestani et al. 2012). The male:female birth ratio of A. axis is about 7:10 ( Mukhopadhayay 2001). Mean birth weight (± SD) is 3.53 ± 0.52 kg (range 2.5–4.7 kg) with mean male fawn weight (3.71 ± 0.47 kg) slightly larger than female fawn weight (3.37 ± 0.47 kg) at birth ( Chapple 1989; Willard et al. 1998; Azad et al. 2005). Average daily weight gains from birth to day 280 were 106.0– 148.2 g /day for males and 105.0– 119.3 g /day for females ( Willard et al. 1998). Fawns regularly are left hidden by does for extended periods, with their mothers returning to allow suckling ( Schaller 1967). Mean duration of nursing ranges from 44 to 69 s based on 382 observations ( Schaller1967). Fawns are known to consume solid foods as early as 3 weeks of age (Khanpara and Vachhrajani 2007), with weaning at 12–20 weeks of age and complete weaning at 6 months of age (Graf and Nichols 1966; Chapple 1989; Azad et al. 2005).
Axis axis can conceive at about 9 months of age under the optimal nutritional conditions of captive rearing ( Raman 1998), but estrus can be delayed as long as 2–3 years under suboptimal nutritional conditions. Estrus occurs after a female reaches 50% of mature body weight. Estrus is typically signaled by continual tail flipping and marked restlessness of females ( Chapple 1989). All males over 1 year of age with hard antlers produced sufficient sperm to breed, with a positive relationship between the testes:body size ratio and quantity of sperm ( Umapathy et al. 2007). Testicular volume mirrors the antler development cycle, with peak testicular volume occurring while males are in hard antler and minimum testicular volumes occurring after antlers are shed (Loudon and Curlewis 1988; Umapathy et al. 2007).
Reproduction.— Breeding periods for Axis axis vary widely and can be classified as aseasonal. Regionally, breeding seasons appear to be influenced primarily by weather and climatic conditions (Delany and Happold 1979; Robbins et al. 1987; Sempéré 1990). Breeding condition of males appears to influence timing of primary breeding season. In its native distribution, northern Indian populations breed in October– April, whereas southern Indian populations breed in April– August ( Raman 1998). Introduced A. axis in Texas commonly breed in May–August, whereas those is Hawaii breed in April–August ( Ables et al. 1977). Estrus lasts for 12–30 h or until a female mates. Reports of the length of the interestrous period are 17–56 days, with 17–21 days being most common. Synchronization of estrous cycles is common among females in the same social unit. When calculated from progesterone profiles in two studies (n = 61), interestrous periods were 18 days averaged ( Chapple et al. 1993; Mylrea et al. 1999). Serum progesterone levels were 2.7–26 nmol/l. One study reported a female entering estrus 11 times in a 12-month period. Gestation lasts 228–239 days ( English 1992), and the average first postpartum estrus occurs 20 days after birth, with as few as 10 days recorded. Twinning is rare, with an average of about 1.03 neonates/female, and litters with triplets have not been documented. Male:female birth ratios are about 1:1 ( English 1992). Longevity in the wild varies with 9–13 years typical, in captive populations longevity can exceed 20 years ( Weigl 2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Axis axis ( Erxleben, 1777 )
| Randel, Charles J & Tomeček, John M 2021 |
Cervus axis maculates
| GROVES, C. P. 2003: 351 |
| KERR, R. 1792: 300 |
Axis axis: R. I. Pocock, 1923:184
| POCOCK, R. I. 1923: 184 |
Cervus (Rusa) axis zeylanicus
| LYDEKKER, R. 1905: 947 |
Hyelaphus maculatus:
| FITZINGER, L. J. 1874: 259 |
Axis maculata ceylonensis:
| FITZINGER, L. J. 1874: 269 |
Axis nudipalpebra:
| FITZINGER, L. J. 1874: 270 |
Axis maculatus:
| JERDON, T. C. 1867: 260 |
Axis maculata:
| GRAY, J. E. 1843: 178 |
Cervus (Axis) major
| HODGSON, B. H. 1841: 914 |
Cervus (Axis) minor
| HODGSON, B. H. 1841: 914 |
Axis aculatus
| JARDINE, W. 1835: 167 |
Cervus nudipalpebra
| OGILBY, W. 1831: 136 |
Cervus axis var. ceylonensis J. B. Fischer, 1829:619
| FISCHER, J. B. 1829: 619 |
Cervus axis var. indicus J. B. Fischer, 1829:619
| FISCHER, J. B. 1829: 619 |
Cervus axis:
| HAMILTON-SMITH, C. 1827: 117 |