Cheiloxena Baly, 1860
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4497.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:7DFD16E7-D628-48DA-AAD6-4AB1E4D679E2 |
|
DOI |
https://doi.org/10.5281/zenodo.5958926 |
|
persistent identifier |
https://treatment.plazi.org/id/2A2D1376-6B70-FF87-FF1A-F9A805AA5B6E |
|
treatment provided by |
Plazi |
|
scientific name |
Cheiloxena Baly, 1860 |
| status |
|
Chiloxena: Gemminger and von Harold 1874: 3233 (misspelling)
Cheiloscena: Selman 1963: 158 (misspelling)
Chiloscena: Selman 1963: 159 (misspelling)
Type species: Cheiloxena westwoodii Baly, 1860 , by original designation and monotypy
Diagnosis. Length 7¯ 15.5 mm; shape elongate, profile not elevated at base of elytra; elytra parallel-sided for basal 2/3, base of pronotum strongly narrowed compared with elytral width at humeri; dorsum dull, with scattered recumbent scale-like setae; eyes generally small, gena 0.3–0.8x eye length; pronotum widest at middle and/or anterior, and width at posterior angles narrower than width at anterior angles; lateral margination of pronotum absent; prosternal process elongate, arched between coxae, apex truncate and laterally expanded; elytra tuberculate, non-striate, but some punctures may be aligned in short rows; epipleuron narrow, gradually contracted from humerus to apex, upper margin obliterated at base; mesosternal process elongate, apex truncate; male tibiae straight; tibial spurs 1+2+2; claw bifid, inner lobe small and acute, <45°; apex of third tarsomere shallowly bilobed; ventrites I and II fused; tegmen with internal median keel; male spiculum relictum absent; female median sclerite present in ovipositor; rectal kotpresse not well-defined.
Description. Length 7–15.5 mm, females larger than males on average but ranges broadly overlapping; body ( Figs 1–20 View FIGURES 1–2 View FIGURES 3–6 View FIGURES 7–10 View FIGURES 11–12 View FIGURES 13–21 ) elongate, length 1.8–2.3x width; head generally deflexed (least so in C. insignis ); pronotal base much narrower than elytral base at humeri, which are prominent; dorsum and venter dark brown to black, with broad recumbent scale-like setae, except C. insignis with recumbent thin setae; profile shallowly convex, but often with prominent tubercles, length 2.6–3.1x height (excluding tubercles).
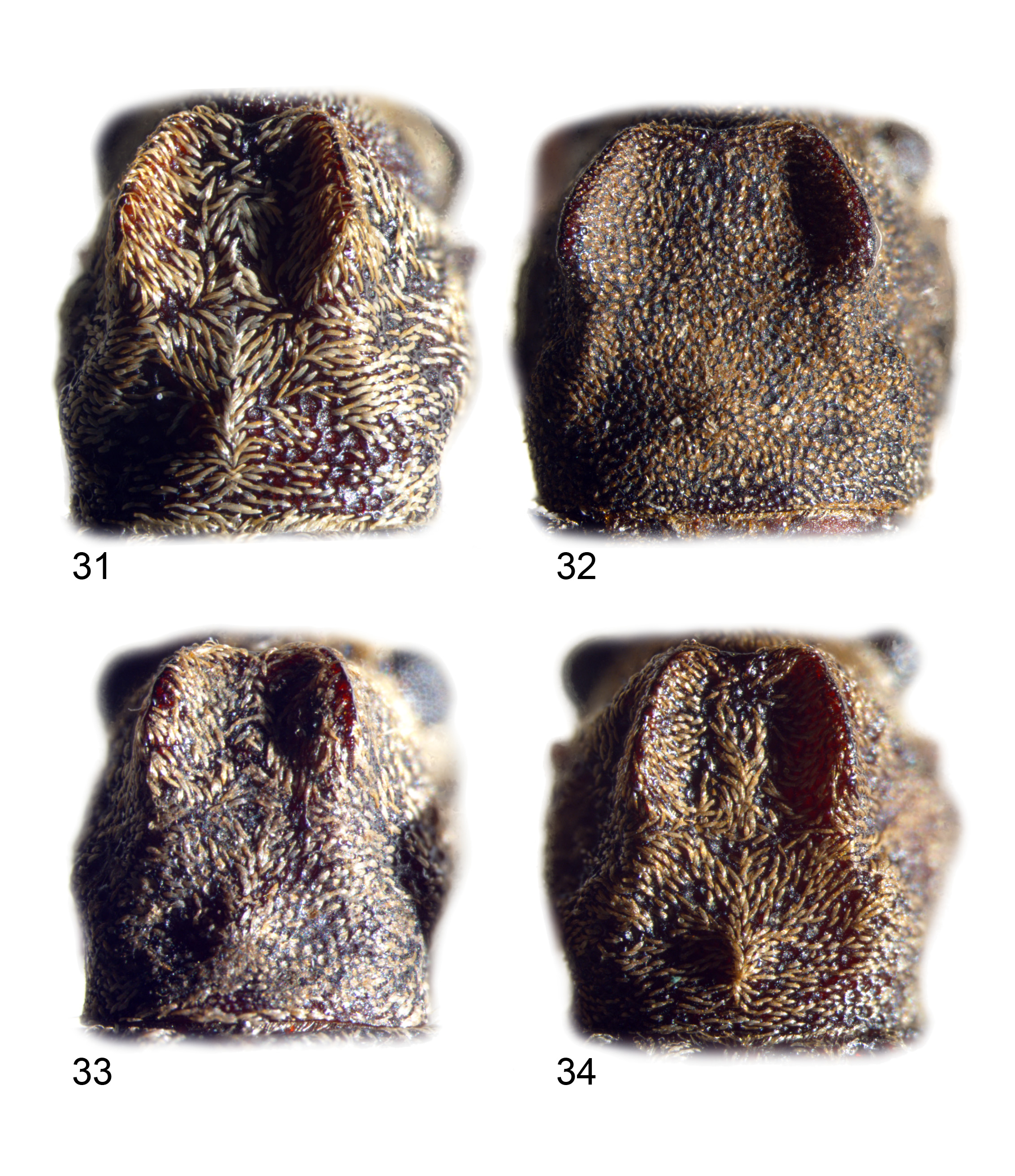
Head ( Figs 1–30 View FIGURES 1–2 View FIGURES 3–6 View FIGURES 7–10 View FIGURES 11–12 View FIGURES 13–21 View FIGURES 22–30 , 35 View FIGURES 35–37 , 38, 39 View FIGURES 38–42 ): head capsule dorsally without deep grooves or ridges; fronto-clypeal sutures obsolete, but frontoclypeus distinguished by narrow impunctate margins delimiting an approximately triangular area, anterior margin truncate to deeply concave, flat or concave between eyes and without elevated apex; vertex and frontoclypeus punctate, punctures larger than ommatidia; eyes small and ovate, length 1.3– 2x width, inner margins shallowly concave, inter-ocular space 2– 4x eye lengths; eye feebly to strongly laterally projecting, if latter, posterior curvature contiguous with short temples, which are constricted to parallel-sided base of head capsule; small trichobothrium present at posterior margin of eye; pregular area smooth and impunctate (normally hidden); gena produced, 0.3–0.8x greatest eye length; antennae 2.5x (male C. insignis ) to 9x (female C. conani ) socket diameters apart, sockets laterally directed; antennae 0.55–0.9x body length; relative sizes of antennomeres variable, but 1 always widest, 2 shortest and quadrate to transverse, 3 longer than 1, antennomeres 8–11 elongate and parallel-sided or short and ovate ( C. insignis ), 3 longest, and 7 or 11 second longest; antennomeres 1–6 relatively shining and sparsely punctured, 8–11 (sometimes also apical half of 7) dull and densely microsculptured; membranous anteclypeus usually produced, smooth and impunctate; labrum transverse, broadest at apex, flat except for declivous apical edge, often only the latter visible, anterior margin shallowly concave to truncate; apical half shining, sparsely punctate, with 2–4 pairs (sometimes asymmetric) of long setae at edge of declivity and fringe of long setae projecting from underside of apical edge, forming tuft at each apicolateral corner; mandibles shining and almost impunctate on apical 1/3–1/2, closely punctured and setose on basal 2/3–1/2; each mandible produced, bent at almost right-angles to two apical teeth; apical maxillary palpomere elongate, fusiform in females, varying from elongate ovate to securiform in males, but in males always with broader truncate apex; preapical maxillary palpomere elongate-triangular, shorter than apical, and narrower; labial palpi similar to maxillary palpi in both sexes, but narrower; mentum transverse, width 2– 3x median length, anterior angles slightly produced.
Thorax ( Figs 1–36 View FIGURES 1–2 View FIGURES 3–6 View FIGURES 7–10 View FIGURES 11–12 View FIGURES 13–21 View FIGURES 22–30 View FIGURES 31–34 View FIGURES 35–37 , 40–42 View FIGURES 38–42 ): pronotum elongate to transverse, width 0.85–1.3x length, broadest at middle, or middle and anterior angles, usually strongly contracted from middle to posterior angles and slightly contracted from middle to anterior angles; disc punctured, uneven, with lateral depressions and/or paired median ridges; anterior edge produced in front of anterior angles, posterior edge truncate to convex, lateral margins slightly convex to strongly toothed or lobed; anterior and posterior edges without distinct beading; lateral carinae absent, or reduced to short ridge between anterior angle and anterolateral tubercle in C. insignis ; anterior angles anteriorly prominent, with small trichobothrium; posterior angles not produced, 90–100°, with small trichobothrium laterally placed; base of elytra not or slightly hollowed to accommodate posterior of pronotum; prosternum flat and punctate between procoxae and head; prosternal process elongate, narrow and strongly arched, with truncate expanded apex; procoxal cavities pear shaped, rounded at inner margin and attenuated laterally; protrochantins narrowly exposed; procoxal cavities closed by insertion of hypomeral lobes into prosternal process; scutellum semi-ovate, flat, apex superimposed on sutural base; elytra broadest at base or basal 2/3, with strongly developed humeri; surface with shiny tipped tubercles and irregular depressions, usually including an arcuate depression in basal half; elytral puncturation irregular, without distinct striae but partially seriate in some C. insignis ; discal punctures deep and vertically walled with transparent shiny bases and pair of shiny tubercles laterally on rim (absent in C. conani ); elytral sutural margin elevated in apical third; elytral epipleuron narrow, width <0.2x elytral width, entirely visible laterally, slightly sinuate, gradually contracted from base to apex but upper margin erased at base, below humeri; mesoventrite entirely visible, well-developed, punctured, anterior without procoxal rests, posterior with elongate and parallel-sided median process strongly raised to truncate apex; wings fully developed, with two closed cells in anal field; metaventrite densely punctured and microreticulate except at midline, transverse, width about 3x length, without femoral plates, anterior with complete margination and without median depression, inner edge of margin not crenulate; metepisternum flat, densely punctured and microreticulate, posterior apex thin, slotting into groove at base of abdomen; all femora clavate, ovate in cross-section, with basal half thin and apical half expanded, with paired longitudinal keels on inner and outer faces of basal half; all tibiae oval in mid cross-section, with abruptly expanded apices; metatibiae with shallow irregular to deep regular lateral longitudinal grooves, defining keels when grooves are deep and regular; metatibiae thin and elongated to short and robust; 1 minute spur at apex of protibia, 2 minute spurs at apices of other tibiae (spurs small and often broken off); all first tarsomeres ventrally with dense setae, mostly apically directed in males, diverging from midline in females; apices of first and second tarsomeres broadly concave; apex of third tarsomere shallowly excavate at middle, depth 0.2–0.1x length of tarsomere; claws bifid, internal tooth acute and about half length external tooth.
Abdomen ( Figs 36, 37 View FIGURES 35–37 , 43–76 View FIGURES 43–51 View FIGURES 52–61 View FIGURES 62–68 ): pygidium (tergite 7) with basal half membranous, apical half densely pubescent and punctured, without median groove, and apex strongly sclerotised; all ventrites punctured and pubescent, densely on ventrite V; all ventrites entirely laterally margined; ventrite I with truncate intercoxal process and without femoral plates; ventrites I and II completely fused; ventrites III–IV generally shorter at midline in male; ventrite V smooth, not medially depressed; male tergite VIII strongly sclerotised with strongly reflexed anterior edge, sternite VIII (spiculum relictum) membranous, not distinguishable; sternite IX (spiculum gastrale) of male Y-shaped, but left branch always distinctly shorter than right branch; male tegmen present, almost enclosing penis, Y- shaped, thinly sclerotised and with distinct internal median keel; penis simple, flattened-tubular and strongly reflexed in apical half (bent at 45–90°, or strongly curved), apex sparsely microspiculate, basal foramen 0.4–0.5x length of penis; vas deferens with long thickened sperm pump; female tergite VIII well-developed, sternite VIII laterally and apically membranous, with elongate basal apodeme; ovipositor with thin sclerite wrapped around base of well-developed paraprocts, which partly enclose basal half of palpi, pair of well-defined elongate proctigers dorsal to these; vaginal palpi 2-segmented, gonocoxite massive, not divided, gonostylus small and conical, apically situated; membranous pad between gonocoxites with elongate but poorly defined sclerite (the median ventral sclerite); spermatheca falciform, surface smooth, with moderately long coiled or uncoiled spermathecal duct; kotpresse present as complete ring of sparse spinules, without well-defined dense dorsal and ventral patches of spinules.
Notes. Sexual dimorphism is slight, however males are easily distinguished by having apically expanded last maxillary palpomeres compared with the simply ovate female last maxillary palpomeres. Males are also smaller on average, with larger cephalic sensory organs (antennae longer and thicker, eyes proportionally slightly larger) and first tarsomeres slightly broader.
In Cheiloxena there is little interspecific variation in ventral structures, such as the thorax and abdominal sclerites, in contrast to other spilopyrine genera such as Macrolema Baly, 1861 , and Spilopyra ( Reid & Beatson 2010a, 2010b). Our species diagnosis is mostly based on variation in antennal structure, tubercles and lobes on the pronotum and elytra, and on the genitalia. As in other Spilopyrinae , interspecific genitalic variation is slight. Molecular study of this genus has not been attempted and is hampered by rarity of the species.
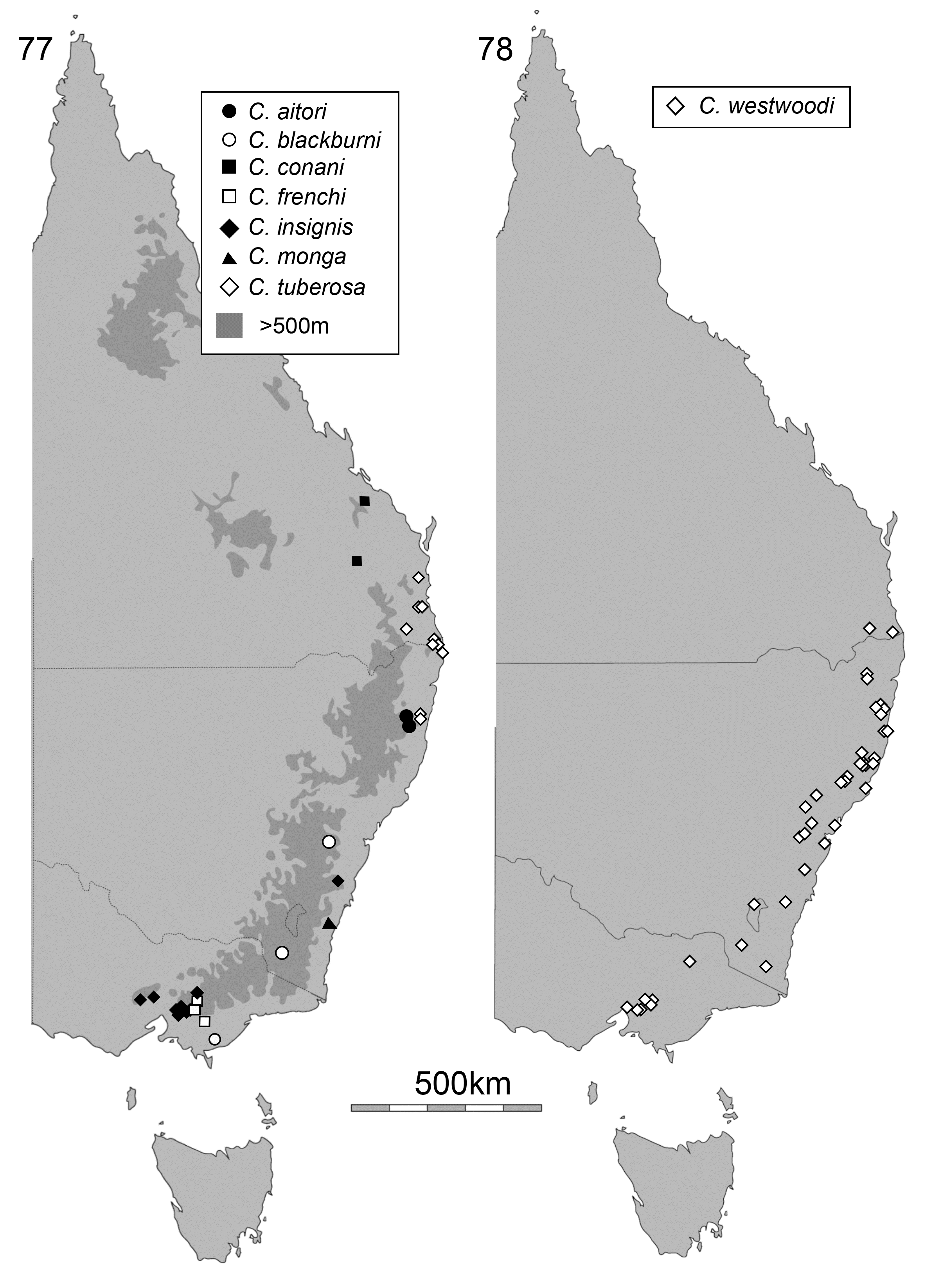
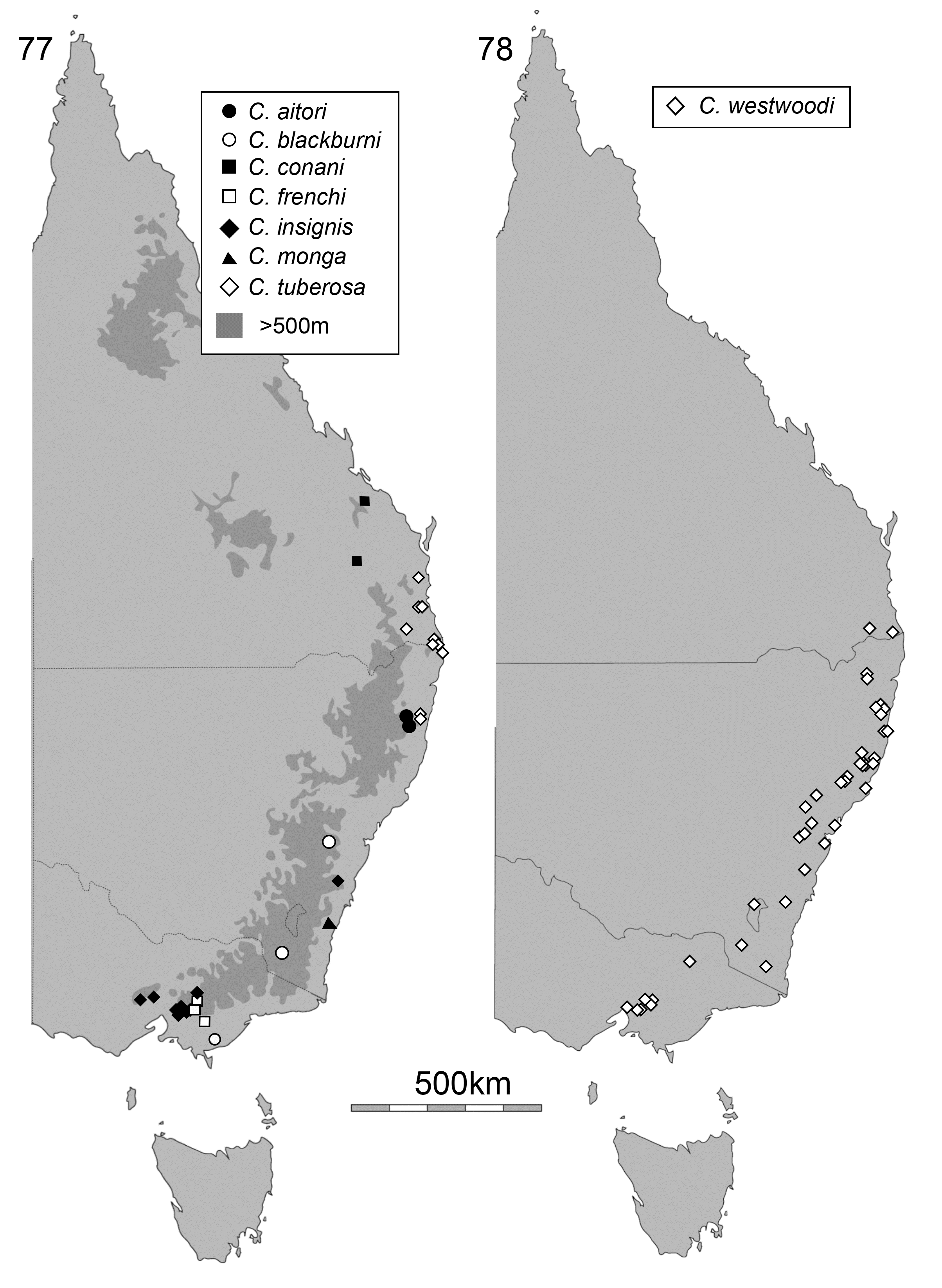
Distribution and biology. Cheiloxena is endemic to Australia, where it occurs on the eastern ranges and coastal plains (2–1400 m elevation) from southern Victoria to south-central Queensland at Kroombit Tops ( Figs 77 View FIGURE 77 , 78 View FIGURE 78 ). It is absent from Tasmania. All species are fully winged and Cheiloxena species are occasionally collected at light. Almost all Cheiloxena specimens have been collected from October to February, especially the summer months of November to January, and the sex ratio is approximately 1:1. All species of Cheiloxena appear to be cryptically coloured and therefore may be hiding on dead branches or in leaf litter during the day.
Cheiloxena species are mostly restricted to temperate or cool temperate forest in southeastern Australia, unlike other Australian spilopyrines, which occur in tropical or subtropical rainforests ( Reid & Beatson 2010a, 2010b, 2011). The life history of Cheiloxena is poorly known. Several specimens of C. westwoodii Baly were collected on Astrotricha latifolia (Araliaceae) View in CoL and kept alive in a laboratory, where they fed on this plant but failed to lay eggs. Astrotricha View in CoL species are generally uncommon in eastern Australia ( Hnatiuk 1990; Anonymous 2017). Feeding on Araliaceae is extremely rare in Chrysomelidae View in CoL , with only three other genera recorded feeding on this family, in Chrysomelinae and Lamprosomatinae View in CoL ( Jolivet and Hawkeswood 1995). Astrotricha View in CoL may be the host of all Cheiloxena species as all localities for the beetle are known to have Astrotricha View in CoL present ( Hnatiuk 1990; Anonymous 2017) and both plant and beetle are absent from Tasmania. However, three specimens of C. frenchae were collected on Lomatia fraseri (Proteaceae) View in CoL and a leaf of this plant was partly chewed by two of them in captivity. The female C. frenchae laid three eggs, each covered in faecal material, but these eggs failed to hatch. A single specimen of the new species similar to C. frenchae was collected from Lomatia arborescens View in CoL in northern New South Wales. The distribution of Lomatia View in CoL is similar to Astrotricha View in CoL but includes Tasmania ( Hnatiuk 1990; Anonymous 2017) and it also occurs in South America ( Stevens 2017). Lomatia View in CoL leaves have long trichomes ( Gonzalez et al., 2004), like Astrotricha View in CoL leaves, and trichomes are known to provide defence against eruciform insect larvae ( Kariyat et al., 2017). Three species of Cheiloxena have also been collected from foliage of Eucalyptus (Myrtaceae) View in CoL , which lacks trichomes, and there are single records on other plants, but all without evidence of feeding. Larvae of Cheiloxena remain unknown. The failure, so far, to find larvae of Cheiloxena may indicate that the larvae have a different biology to other spilopyrine genera, all of which are known, however larvae of this subfamily are generally nocturnal and rarely collected ( Bohumiljania: Reid & Beatson 2011 ; Dorymolpus: Elgueta, Daccordi & Zoia 2014; Hornius: Jerez 1996 ; Macrolema: Reid & Beatson 2010a ; Richmondia : undescribed, but reared by one of the authors (CAMR); Spilopyra: Reid & Beatson 2010b ; Stenomela: Jerez 1996 ).
Three of the species of Cheiloxena have been fairly commonly collected, the other five are represented by 23 specimens in collections. Cheiloxena blackburni Reid, 1992 , is only known from a small amount of old material (most recent dated specimen = 1935), without detailed locality, and may reasonably be considered ‘Endangered’ in conservation status (International Union for Conservation of Nature 2012). Records of several identifiable Cheiloxena species are available from detailed photographic records posted on websites. The amateur photographer-naturalist community provides a valuable service posting images of these rarely seen animals, for which we are very grateful.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cheiloxena Baly, 1860
| Reid, C. A. M. & Beatson, M. 2018 |
Cheiloxena
| Baly, 1860 : 255 |
Chiloxena: Gemminger and von Harold 1874 : 3233
| Harold 1874 : 3233 |