Cambarus (Puncticambarus) taylori, Loughman, Zachary J., Henkanaththegedara, Sujan M., Fetzner Jr, James W. & Thoma, Roger F., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4269.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:370D38B9-5FED-4AE6-915D-3CB74F044C71 |
|
DOI |
https://doi.org/10.5281/zenodo.6028570 |
|
persistent identifier |
https://treatment.plazi.org/id/BB373BDC-ED1F-4802-8E4D-7CB14D7C2FA1 |
|
taxon LSID |
lsid:zoobank.org:act:BB373BDC-ED1F-4802-8E4D-7CB14D7C2FA1 |
|
treatment provided by |
Plazi |
|
scientific name |
Cambarus (Puncticambarus) taylori |
| status |
sp. nov. |
Cambarus (Puncticambarus) taylori View in CoL , new species
Figures 9–11 View FIGURE 9 View FIGURE 10 View FIGURE 11 , Table 6
Cambarus robustus Girard, 1852:90 View in CoL [in part];— Taylor and Schuster, 2004:103, Figs. 74A–B, 75. Cambarus (Puncticambarus) robustus View in CoL .—Hobbs, 1969:101, Figs. 1 View FIGURE 1 c, 13a, 17o [in part]; 1974:21, Fig. 87 [in part]; 1989:27, Fig. 104 [in part].
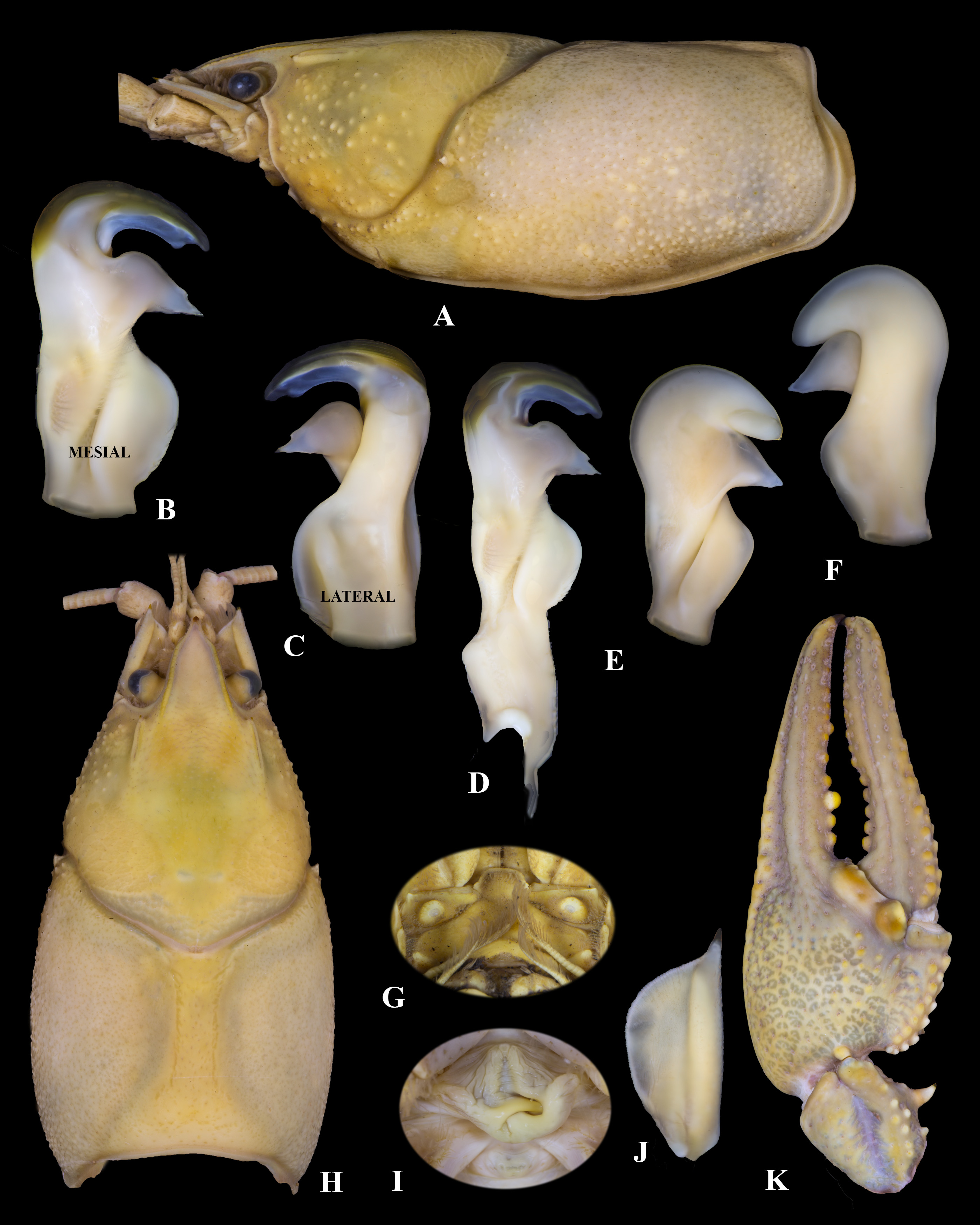
Diagnosis. Body and eyes pigmented. Rostrum narrow, not excavated, gently directed vertically, margins not thickened, subparallel, converging at acumen. Acumen distinctly triangular with prominent dorsally directed spiniform tubercle at terminus. Areola 3.6–6.8 (x ‾ = 4.6, n = 26, SE = 0.8) times as long as wide with 6–8 (mode 6) punctations across narrowest point. Pronounced cervical spines present (100%; n = 26). Mandibular, branchiostegal, and orbital regions of carapace with well-developed tubercles. Postorbital ridges developed; pronounced spiniform, dorsally directed tubercle present in all age classes. Suborbital angle acute. Total carapace length 1.8–2.1 (x ‾ = 1.9, n = 27, SE = 0.1) times longer than width. Form I and II males possessing hook on ischium of third pereopods only; hook gently curved at apex, overarching basioischial joint in Form I males, not reaching basioischial joint in Form II males; hooks not opposed by tubercle on basis. Mesial surface of chelae with two rows of tubercles; mesial most row with 6–13 (x ‾ = 8.2, n = 27, SE = 2.1) tubercles, second dorsal row with 6–9 (x ‾ = 7.2, n = 27, SE = 1.9) tubercles. Tubercles normally not extending onto upper body of palm. Ventral surface of chelae with 2–6 (x ‾ = 9.1, n = 48, SE = 1.9) subpalmar tubercles near the dactyl/propodus junction; when more than one subpalmar tubercle present, tubercles oriented in a straight line parallel to the mesial margin. Dorsal longitudinal ridge of dactyl consisting of several well-developed, pronounced, scattered tubercles. Dorsomedian ridge of fixed finger of propodus pronounced. Well defined lateral impression at base of fixed finger. Dactyl and fixed finger with sharp, corneous, subterminal tip. Form I male palm length 65.2–65.3% (x ‾ = 65.2%, n = 4, SE = 0.2%) palm width, Form I male palm length 26.5–27.5% (x ‾ = 27.0%, n = 4, SE = 0.7%) of total propodus length; female dactyl length 57.5–63.9% (x ‾ = 60.7%, n = 15, SE = 1.9%) of total propodus length. First pleopod of Form I male with short terminal elements. Central projection not tapering distally; recurved>90° to main shaft of gonopod, with distinct subapical notch. Mesial process directed>90° to shaft, bent basolaterally; inflated basally, tapering to distinct caudal point equal to the terminance of central projection with notch. Annulus ventralis immovable; distinctly asymmetrical posteriorly; anterior portion with median trough leading to strongly sculptured central fossa; exaggerated “S” bend in sinus terminating at caudal edge.
Description of Holotypic Male, Form I. ( Fig. 9 View FIGURE 9 A–D, G, H, J, K, Table 6).––Body not compressed dorsoventrally ( Fig. 9 View FIGURE 9 A); carapace posterior to cervical groove wider than abdomen. Carapace depth less than carapace width at dorsalcephalic margin of cervical groove. TCL 56.0 mm; PCL 46.5 mm. Areola 5.1 times longer than wide, with 8 punctations across narrowest part ( Fig. 9 View FIGURE 9 H); length of areola 36.5% of TCL (43.9% PCL). Rostrum not excavated; margins slightly thickened, subparallel and continuous to base of acumen; floor of rostrum with numerous punctations. Rostrum 1.8 times longer than wide and lanceolate. Acumen ending in dorsally directed corneous tip ( Fig. 9 View FIGURE 9 H). Postorbital ridges well developed, terminating in pronounced, anterior directed, spiniform, tubercles. Suborbital angle acute, with small tubercle ( Fig. 9 View FIGURE 9 H). Pronounced cervical spine present. Mandibular, branchiostegal, and orbital regions of carapace ornamented with well-developed tubercles; greatest tubercle density in hepatic region. Abdomen supraequal in length to carapace, pleura rounded cephaloventrally, angled distoventrally. Lateral margin of terga angulate; lateral margin of second pleuron deeply furrowed. Cephalic section of telson with 2 large spines in each basolateral corner. Proximal podomere of uropod with distal spine on mesial lobe; mesial ramus of uropod with median ridge ending distally in spine, not overreaching margin of ramus; laterodistal spine pronounced. Distal margin of proximal segment of lateral ramus of right uropod having 18 immovable, small spines and one lateral, large, movable spine. Cephalomedian lobe of epistome subrectangular, zygoma moderately arched ( Fig. 9 View FIGURE 9 G); cephalolateral margins thickened, forming sharp angle at junction with endostyle; body of epistome lacking prominent cephalomedian fovea ( Fig. 9 View FIGURE 9 G). Antennal scale broadest anteriorly; lateral margin thickened, terminating in large corneous spine; setiferous on mesial margin ( Fig. 9 View FIGURE 9 J). Right antennal scale 9.5 mm long, 4.1 mm wide ( Fig. 9 View FIGURE 9 J). Tip of right antenna reaching middle of telson when adpressed. Mesial surface of left chela with 2 well-formed rows of tubercles; mesial most row with 10 tubercles, dorsal row with 7 tubercles ( Fig. 9 View FIGURE 9 K). Several disorganized tubercles present on mesial surface of palm near dactyl/propodus junction. Palm length 65.5% of palm width; depth of palm 15.4 mm. Ventral surface of palm containing 4 subpalmar tubercles forming a line. Dorsal longitudinal ridges of dactyl well developed and possessing moderate sized tubercles ( Fig. 9 View FIGURE 9 K); dactyl terminating in large corneous spine. Dorsomedian ridge of propodus finger pronounced. Well-defined lateral impression at base of opposable propodus; numerous setiferous punctations present. Dactyl and fixed finger of propodus with sharp, corneous tip. Carpus with prominent dorsal furrow ( Fig. 9 View FIGURE 9 K) and seven weak dorsomesial tubercles; remaining surface with some setiferous punctations; mesial margin with large, procurved spine at about mid-length, and reduced proximal spine. Distodorsal surface of merus with three spiniform tubercles; ventrolateral ridge with 2 small spines and large, corneous distal spine; ventromesial ridge with eight well-developed spines; ventrolateral margin of ischium with two small, spiniform tubercles. Hook on ischium of third pereopods only; hook gently curved at apex, overarching basioischial joint, not opposed by tubercle on basis. Form I gonopod as described in diagnosis ( Fig. 9 View FIGURE 9 B–D); tip reaching anterior margin of fourth caudomesial boss.
Description of Allotypic Female. ( Fig. 9 View FIGURE 9 I, Table 6).––Differing from holotype in following respects; carapace depth (22.1 mm) 77.3% of carapace width (28.6 mm); TCL 53.9 mm, PCL 44.4 mm. Areola length 36.4% of TCL (44.4% of PCL), 5.7 times as long as wide. Rostrum 1.9 times longer than wide. Abdomen length 52.2 mm. Ten tubercles on mesial most row of chelae, second row with seven. Palm length (14.2 mm) 69.6% of palm width (20.4 mm); depth of palm 12.2 mm. All measurements and counts from right chela. Antennal scale 8.0 mm long, 3.8 mm wide. Annulus ventralis as described in diagnosis ( Fig. 9 View FIGURE 9 I); width of postannular sclerite half the total width of annulus ventralis; first pleopods uniramous, reaching central region of annulus ventralis when abdomen flexed.
Description of Morphotypic Male, Form II. ( Fig. 9 View FIGURE 9 E–F, Table 6).––Differing from holotype in the following respects: Carapace depth (25.1 mm) 84.5% of carapace width (29.7 mm); TCL 53.4 mm and PCL 44.0 mm. Areola length 36.3% of TCL (44.1% of PCL), 5.5 times longer than wide. Rostrum with margins subparallel to base of acumen; ventrally deflected; length 2.0 times width. Abdomen 51.6 mm long. Mesial row of tubercles on palm of chela with seven tubercles; second row with six. Palm length (13.9 mm) 68.8% of palm width (20.2 mm). All measurements and counts from right chela. Antennal scale 9.7 mm long, 3.9 mm wide. Gonopods reaching anterior margin of 4th pereopod caudomesial boss. Central projection curved at>90° to shaft, with complete apex; rounded ( Fig. 9 View FIGURE 9 E–F). Mesial process tapered, bulbous, directed basiolaterally. Hook on ischium of third pereopod small, not reaching basioischial joint.
Size. Form I male (n = 4) TCL ranges in size from 45.9–56.0 mm (PCL 37.4–46.5 mm), x ‾ = 50.9 mm. Form II male (n = 9) TCL ranges from 39.6–47.5 mm (PCL 26.8–47.5 mm), x ‾ = 46.4 mm. Female (n = 26) TCL ranges from 32.4–53.4 mm (PCL 26.4–42.9 mm), x ‾ = 43.3 mm. The largest specimen examined was a Form I male with a TCL of 56.0 mm (PCL 46.5 mm).
Color in life. Carapace ground color light brown to yellow to orange-brown; posterior margin of carapace light blue to green. Hepatic and antennal region of carapace ornamented with cream, olive, or tan tubercles. Postorbital ridge color same as carapace, to orange-brown. Rostrum margins and acumen cream to orange, orange-brown or tan. Cephalic section of carapace immediately anterior to and including cervical groove black, forming weak saddle; mandibular abductor scars ranging from cream to light-brown. Lateral margin of antennal scale cream to light brown; body of antennal scale blue-brown to cream. Antennal flagellum and antennules green-brown, with olivaceous hue; dorsal surface of lamellae tan to brown; ventral surface light green to olivaceous. Dorsal surface of chelae gray-blue, light blue, green-blue, or green; with no mottling; mesial row, second dorsal row, and dorsal surface of dactyl tubercles cream, tan or orange. Denticles on opposable surfaces of fingers yellow, white, or tan. Ventral surface of chelae cream or tan. Dorsal surface of carpus light blue, green-blue, or olivaceous gray; region adjacent to and including furrow green-brown to green; carpus spine cream. Merus green-brown, gray, or olivaceous brown. Podomeres of pereopods light blue, cream, or gray-blue; joints of pereopod podomeres pink. Dorsal and dorsolateral surface of abdomen light blue, blue-gray, aqua, or green-blue; tergite margins brown to reddish brown; abdomen lacking dorsal stripe. Uropods same colors as abdomen. Ventral surface of abdomen and carapace cream. Dorsal ridge of Form I gonopod central projection amber; body of central projection, gonopod and mesial process tan. Form II gonopod and all associated processes cream. Cephalic portion of annulus ventralis pink to pink-cream; ridge of fossa pink; caudal region of annulus ventralis ranges from pink to cream colored.
Type locality. Fourmile Fork at Houston Road (KY 1114) crossing, 1.9 km (1.2 mi) southeast of Turkey, Breathitt County, KY, 37.46333/-83.49893 (WGS-84) ( Fig. 11 View FIGURE 11 , star). At this site, Fourmile Fork is 8–15m wide, and consists of a series of riffles, runs, and pools. Substrates utilized by C. taylori included large boulders and large slabs, as well as leaf packs. Water depth ranged from 0.2–1.0 m deep. Here, the type series was collected, along with several additional specimens, on 25 October 2014 by S. Bell, Z. Dillard, L. Sadecky, R. K. Scott, K. R. Loughman, C. Z. Loughman and ZJL. Orconectes cristavarius is also abundant at this location.
Disposition of types. The holotype, allotype, and morphotype are deposited in the North Carolina Museum of Science ( NCSM), Raleigh, NC. (catalogue numbers NCSM 27209, 27210 and 27211, respectively) . Paratypes are deposited in the USNM, Washington D.C. ( USNM 1422181 View Materials ) .
Range and specimens examined. Cambarus taylori ( Fig. 10 View FIGURE 10 ) is endemic to the Cumberland Mountains region, and specifically to the Middle Fork Kentucky River and its tributaries in Breathitt, Harlan, and Leslie counties, Kentucky, USA ( Fig. 11 View FIGURE 11 ). The senior author was unsuccessful in collecting C. taylori in the North Fork Kentucky River at its confluence with the Middle Fork. Cambarus hazardi was the only tertiary burrowing Cambarus encountered at that location. Within the Middle Fork basin, C. taylori has been collected in Beech Creek, Cutshin Creek, Cow Creek, Grays Fork, Greasy Creek, Hurst Fork, Laurel Creek, Turkey Creek and the Middle Fork mainstem.
All of the following collections, with the exception of the previously discussed type series, are either housed in the Branley A. Branson Museum at Eastern Kentucky University (denoted with EKU), the United States National Museum (denoted with USNM), or the West Liberty University Astacology Collection (denoted with the prefix WLU). The following abbreviations occur in the text: Cr. = Creek; R. = River; Frk. = Fork ; UNT = Un-named tributary; ZJL14—Collectors included ZJL 2014 field crew which consisted of S. S. Bell, Z. W. Dillard, N. M. Sadecky, L. K. Sadecky, E. Tidmore and E. M. Tennant.
KENTUCKY: Breathitt Co. (1.) WLU 2095 View Materials , Fourmile Frk., 37.4772/-83.4962, 11 June 2014, 7 F, 4 IIM ZJL14; NCSM 27209, 27210 , 27211; USNM 1422181, 25 , October 2014 , TYPE SERIES COLLECTION, 6 F, 2 IM, 5 IIM, ZJL14. C. Z. Loughman and K. R. Loughman. Clay Co. (2.) WLU 2298 View Materials , Grays Frk., 37.2001/-83.4199, 25 October 2014, 7 F, 5 IIM, ZJL14. Harlan Co. (3.) WLU 2100 View Materials , Middle Frk . Kentucky R., 37.0232/-83.4199, 8 June 2014, 1 IIM, ZJL14. Leslie Co. (4.) EKU 3353 View Materials , Middle Frk . Kentucky R., 21 April 1978, 4 IIM, D.L. Batch. (5.) USNM 147032 View Materials , Middle Frk . Kentucky R., 19 October 1974, 6 F, 10 IIM, 1 IM, R.W. Bouchard and J.W. Bouchard. (6.) USNM 147034 View Materials , Middle Frk . Kentucky R., 19 October 1974, 3 F, 6 IIM, R.W. Bouchard and J.W. Bouchard. (7.) WLU 2088 View Materials , Wooton Cr., 37.1663/-83.2843, 10 June 2014, 2 F, ZJL14. (8.) WLU 2090 View Materials , Beech Frk., 36.93985/ -83.390, 10 June 2014, 2F, ZJL14. (9.) WLU 2092 View Materials , Wolf Cr., 37.1080/-83.2943, 8 June 2014, 1 IIM, ZJL14. (10.) WLU 2094 View Materials , Greasy Cr., 36.9985/-83.2956, 8 June 2014, 5 F, 2 IIM, ZJL14. (11.) WLU 2099 View Materials , Laurel Cr., 36.9727/-83.3203, 8 June 2014, 7 F, 1 IIM, ZJL14.
Habitat and life history notes. Cambarus taylori occurs in second through fourth order streams with cobble and boulder substrates. Like C. guenteri , C. taylori is most frequently encountered under large slab boulders in both runs and riffles, although pools containing large slab boulders can also harbor abundant populations of C. taylori . It has not been found in smaller order high gradient streams. Larger order moderate gradient streams appear to be the preferred habitat of C. taylori . Equal numbers of animals occurred in both riffles and runs (ZJL, personal observation).
Very little is known about the life history of C. taylori . Form I males have only been collected in October; Form II males have been collected in June and October. Females exhibited active glair glands in June 2014 (ZJL, personal observation). Ovigerous females or females carrying young have never been collected. Animals collected in late October 2014 exhibited exoskeletons with little if any bioaccumulation, suggesting a recent mass molt had occurred in the weeks prior to collection. Juveniles have been collected in both June and October. Future research should focus on elucidating the life history of this species.
Conservation status. Cambarus taylori should be listed as threatened (T) using American Fisheries Society criteria ( Taylor et al. 2007), and ranked G2 using global conservation criteria for conservation listing due to limited range Master (1991). Cambarus taylori should be listed as vulnerable (VU) using International Union for the Conservation of Nature (IUCN 2001) criteria due to its narrow distribution and a reduction in range caused by anthropogenic stressors, specifically coal mining activities (ZJL personal observation).
Crayfish associates. Cambarus taylori has been collected along with Cambarus (Depressicambarus) cf. sphenoides , Cambarus (Jugicambarus) aff. dubius and Orconectes (Procericambarus) cristavarius .
Variation. Based on material available, C. taylori is highly similar in both morphology and coloration across all age groups and sexes. Occasionally juveniles appear more spinose than adults, specifically in the hepatic region of the cephalothorax. Adults demonstrate little morphological variation within and among sites, with rostrum shape being the only occasional noticeable difference within populations. Form I male rostrum shape varies from narrow to lanceolate. Coloration, like morphology, is also consistent both within and among populations throughout the Middle Fork basin. Chelae and abdomen coloration can range from green, blue-gray to aqua, but normally is a single consistent set of colors within populations.
Relationships and comparisons. Cambarus taylori is placed in the subgenus Puncticambarus based on its elongate chelae, its broad, densely punctate areola (2.1 to 6.2 times as long as broad), and the presence of a subapical notch on the Form I male gonopod (Hobbs 1969; Cooper 2001). Among described members of the subgenus, C. taylori is similar to C. guenteri , C. hazardi , C. veteranus Faxon, 1914 and C. callainus .
Differences among C. taylori , C. guenteri and C. hazardi are discussed in the previous relationships and comparison sections in the descriptions above. Cambarus taylori , like both C. callainus and C. veteranus , possesses a lanceolate rostrum and pronounced cervical spines. Cambarus taylori can be differentiated from both species by the presence of a second dorsal row of tubercles on the mesial margin of the palm compared to the lack of this row in C. callainus and C. veteranus , a more broad rostrum compared to the narrow rostrum of C. callainus and C. veteranus and the palm length/palm width ratio. Cambarus taylori palm length/palm width ratio is>70%, compared to both C. callainus and C. veteranus which have a palm length/palm width ratios <70%. Coloration in life can also be used to differentiate these taxa. In both C. callainus and C. veteranus , coloration is dominated by aquamarine and blues, while in C. taylori , the cephalothorax is less blue and more olivaceous green and brown. Additionally, both C. callainus and C. veteranus in life have rostra that are bordered in crimson red or orange, respectively, and antennae that are either crimson red ( C. callainus ) or reddish orange ( C. veteranus ). Cambarus taylori antennae are a nondescript blue-gray. Cambarus callainus can be found in Kentucky, but does not occur sympatrically with C. taylori , and is restricted to the Upper Levisa Fork in the Big Sandy basin.
Three additional members of the subgenus Puncticambarus occur in Kentucky which are easily differentiated from C. taylori . Both C. cumberlandensis and C. bunting are endemic to the upper Tennessee and Cumberland River watersheds and do not occur naturally within the Middle Fork Kentucky River watershed ( Taylor & Schuster 2004). Both species differ from C. taylori by possessing rostral spines ( C. cumberlandensis ) or tubercles ( C. bunting ), which C. taylori lacks. Cambarus theepiensis also does not occur in the Middle Fork Kentucky River basin, and is limited in Kentucky to the greater Big Sandy catchment. Cambarus theepiensis can be differentiated from C. taylori by its noticeably broader rostrum with thickened margins. Cambarus theepiensis also lacks a cervical spine, which C. taylori possesses.
Etymology. It is our honor and privilege to name this crayfish after Dr. Christopher A. Taylor from the Illinois Natural History Survey. Dr. Taylor has been one of the most active crayfish researchers in the United States for the past two decades and a leader in crayfish conservation, co-authored the seminal work on Kentucky’s crayfishes, Crayfishes of Kentucky, and has been instrumental in bringing the conservation concerns of North America’s crayfishes to light with his many publications. The common name Cutshin Crayfish is in reference to Cutshin Creek watershed, which harbors the species.
Common name. Cutshin Crayfish
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cambarus (Puncticambarus) taylori
| Loughman, Zachary J., Henkanaththegedara, Sujan M., Fetzner Jr, James W. & Thoma, Roger F. 2017 |
Cambarus robustus
| Taylor 2004: 103 |
| Girard 1852: 90 |