Nematomenia brasiliensis, Cobo & Kocot, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4933.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:303F97F8-463C-4A52-B5D7-28154E492493 |
|
DOI |
https://doi.org/10.5281/zenodo.4558033 |
|
persistent identifier |
https://treatment.plazi.org/id/A99DF88C-93AD-45EB-AD5A-595EC665D27A |
|
taxon LSID |
lsid:zoobank.org:act:A99DF88C-93AD-45EB-AD5A-595EC665D27A |
|
treatment provided by |
Plazi |
|
scientific name |
Nematomenia brasiliensis |
| status |
sp. nov. |
Nematomenia brasiliensis View in CoL sp. n.
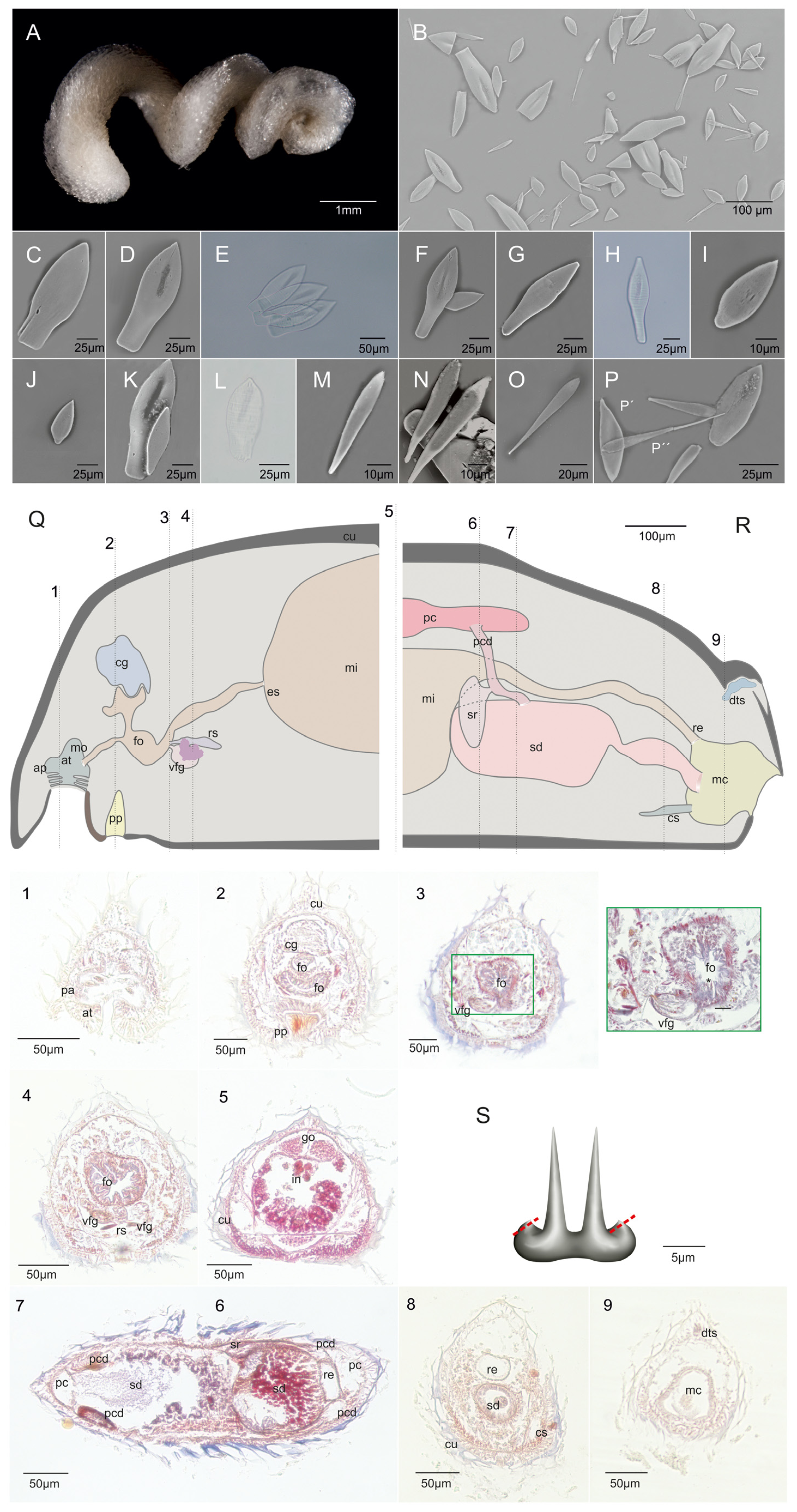
( Figure 5 View FIGURE 5 , Tables 2 View TABLE 2 , 4 View TABLE 4 )
Type material. Holotype: ZSM Mol 20171267 (Zoologische Staatssammlung München). Serial sections (12 slides) and sclerites (one SEM stub, two slides). Brazil Basin, DIVA 3 Me 79/1 area 2, station 561 (26º 34.78’S, 35º 13.90’W), 4484.7 to 4503 m depth. GoogleMaps
Derivatio nominis. From Latin brasiliensis , due to its origin from the Brazil Basin
Diagnosis. Robust animal. Very scaly, with six different types of leaf-shaped scales, two types of pallet-shaped scales, and two different types of scales of the pedal groove. With a sharp dorsal keel and a slight posterior lobe. Atrium with simple papillae. Flat pedal fold. Monoserial radula with at least two curved denticles per tooth. Dorsal foregut gland. Ventrolateral foregut glands of type A, blister-shaped. With one dorsoterminal sensory organ. With copulatory stylets. With seminal vesicles.
Description. Habitus: Long and robust animal (6.95 mm long, 0.55, 0.45, 0.35 mm wide in the anterior, middle, and posterior regions respectively), triangular in cross-section due to the presence of a tegumental dorsal keel. Specimen coiled in a spiral. The sclerites give the specimen a solid, shiny, and scaly appearance. Yellowish preserved in 96 % ethanol. The pedal groove and the common atrio-buccal opening are externally evident. The body ends are pointed and there is a widening (lobe) anterior to the end of the body ( Figure 5 A View FIGURE 5 ). The coiled arrangement in which the specimen was found suggests that it was epizooic on some cnidarian (although no cnidoblasts were found in the digestive tract nor could any of the remains found in the gut could be identified as cnidarian).
Mantle: The epidermis is formed by high rectangular cells, among which are glandular cells. These glandular cells are somewhat wider than the regular cell type and with larger nuclei. Epidermal papillae are absent. The thin cuticle (12.5 to 22.5 μm) shows very marked insertion holes of the scales, which are in a single layer except in the keel region, where two layers of superimposed insertion appear. With six different types of leaf-shaped scales, two types of pallet-shaped scales, and two types of pedal scales: 1) Petiolate leaf-shaped scales with a broad, truncated base and a central depression ( Figure 5 B, C, D, E View FIGURE 5 ) together with the following variety are the largest (108.6 to 145.5 μm long, 34.5 to 58.2 μm wide in the middle region, although most are around 123 μm long, 41 μm wide) and the most abundant variety. They are curved relative to the base (which is inserted into the cuticle). 2) Petiolate leaf-shaped scales with a narrow rectangular truncated base and central depression ( Figure 5 B, F, G, H View FIGURE 5 ). These are like the previous type but narrower (124 to 136.5 μm long, 34.1 to 52 μm wide in the middle region). 3) Leaf-shaped scales with central excavation ( Figure 5 B, K, L View FIGURE 5 ), which are considered a different variety because of their shape and size (69.6 to 75 μm long, 25 to 29.8 μm wide in the middle region), but could be lanceolate leaf-shaped scales that have not yet developed. 4) Leaf-shaped scales with a central short peduncle ( Figure 5 B, I View FIGURE 5 ) that are almost oval (40.6 to 42.78 μm long, 21.8 to 22μm wide in the middle region). 5) Leaf-shaped scales with a short, slightly offcenter peduncle ( Figure 5 J View FIGURE 5 ) that are smaller (41.07 to 82.8 μm long, 20.05 to 31.8 μm wide in the middle region) and less abundant than the previous types. 6) Pallet-shaped scales with a depression in the broadest region ( Figure 5 B, M, N View FIGURE 5 ) that appear mainly in the posterior ventral region of the body and are somewhat longer and narrower than the leaf-shaped scales (52.5 μm long, 9.63 μm wide in the middle region). 7) Pallet-shaped scales with a long and acicular proximal end ( Figure 5 B, O View FIGURE 5 , P’’), which are the least abundant and the most fragile sclerites in this species (95.86 μm long, 7.83 μm wide in the middle region, 2.62 μm wide at the tip). 8) Two types of pedal groove scales: a) short, wide knife-shaped scales (54 to 66 μm long, 15 to 20 μm wide; Figure 5 View FIGURE 5 P’) and b) elongate, knife-shaped scales (62 to 67 μm long, 10 to 11.5 μm wide; not shown).
Pedal groove and mantle cavity: The pedal pit (20 μm long, 32.5 to 37.5 μm wide, 50 μm high) is densely cili-ated with a muscular dorsal wall ( Figure 5 View FIGURE 5 Q-2). It results in a laminar pedal fold (30 to 35 μm wide, 2.5 μm high), which remains constant in size and shape throughout the body and does not enter the mantle cavity. The anterior pedal glands are bulky and barely separate from the ventral wall.
The mantle cavity has a small sub-terminal opening (60 μm wide) ( Figure 5 R View FIGURE 5 ). It is large (82 μm long, 77.5 to 80 μm wide, 60 to 102 μm high) and forms an internal ventro-anterior sac (50 μm long, 30 to 32 μm in diameter) above which the spawning ducts end and in which the copulatory stylets are located. The rectum opens into the dorsal region of the cavity. There are no clear respiratory folds, but the cavity wall has slight folds with cells that appear to be ciliated.
Digestive system: The mouth opens in the center of the common atrio-buccal cavity ( Figure 5 Q View FIGURE 5 ). The narrow foregut is circular in cross-section (15 μm long, 20 to 22 μm in diameter), and very glandular. It becomes wider and runs parallel to the pedal groove (30 μm long, 50 μm wide, 22.5 μm high), surrounded by a thin layer of circular and longitudinal musculature (<2.5 μm thick). In the central region of the foregut, a dorsal glandular pouch (45 μm long, 20 to 40 μm wide, 25 μm high; Figure 5 View FIGURE 5 Q-2) opens through a narrow duct (5 μm in diameter). After the disappear-ance of this dorsal gland, the foregut is lengthened dorso-ventrally (20 μm long, 20 to 25 μm wide, 35 to 37.5 μm high) and then regains its circular cross-sectional shape (25 to 30 μm in diameter), but with a thicker muscular layer (5 to 5.5 μm thick) in the radular region ( Figure 5 View FIGURE 5 Q-3).
The radula is monoserial, with teeth formed by a broad, rounded base with a slight central depression (14 μm wide, 6.6 to 7 μm high laterally, and 3.3 to 4 μm high in the middle region) bearing at least two denticles. The recon-struction of the radula ( Figure 5 S View FIGURE 5 ) was made based on a fragmented tooth. Two denticles were clearly seen. They are long and slightly curved apically where they are thinner (10 to 11μm long and 0.8 to 2 μm wide). At each side, the base two ribs were observed, suggesting the existence of neighboring denticles ( Figure 5 S View FIGURE 5 ).
The ventrolateral foregut glands are of type A. They are blister-shaped: formed by two short and wide ducts (25 to 30 μm in diameter; Figure 5 View FIGURE 5 Q-3, 4) with inner musculature and with extraepithelial gland cells opening into the ducts in their anterior region. The gland opens into the foregut laterally as two independent ducts. Posteriorly bent necks of the glandular cells were not observed ( Pararrhopalia - type).
The esophagus is wider than the foregut along most of its length (95 μm long, 35 to 87.5 μm in diameter). It is directed dorsally at an angle and connects to the mid-anterior region of the midgut via a sphincter (<10 μm aperture). The midgut has neither a caecum nor serial lateral constrictions. The rectum is circular in cross-section (22.5 μm in diameter) and terminates mid-dorsally into the mantle cavity.
Nervous system and sense organs: The nervous system includes a large cerebral ganglion (55 μm long, 75 μm wide, 45 to 60 μm high) that is circular in cross-section ( Figure 5 View FIGURE 5 Q-2). It is located directly above the dorsal gland of the foregut with two small (35 μm long, 12.5 μm diameter) lateral ganglia almost fused to its ventral region, from which a pair of nerve cords emerge. Remains of a pair of pedal ganglia were observed on both sides of the pedal pit. In the radular region of the foregut there are two pairs of buccal ganglia, one dorsal and another ventral, but these were poorly preserved and could not be delimited with precision.
The atrium has numerous simple or pedunculate papillae (10 μm long, 3 to 5 μm wide; Figure 5 View FIGURE 5 Q-1). Situ-ated laterally in the atrium there are also some bilobed papillae (15 to 17.5 μm in diameter), with evident glandular content that is stained an intense red color with Mallory’s Trichrome. The atrium is surrounded by well-developed musculature.
Between the cuticle and the epidermis, in the posterior region of the body (at the end of the posterior lobe), a group of cells (35 μm long, 25 μm wide, 10 μm high) was located and, based on their arrangement and position, could be interpreted as a dorsoterminal sensory organ ( Figure 5 View FIGURE 5 R-8). However, the connection of this cellular package to the outside is not obvious.
Gonopericardial system: The studied specimen has well-formed gonads, with numerous oocytes. It appears that in some sections, in the central region of the gonad, there are some spermatozoa, but these cells could not be clearly characterized. The gonads continue as two wide gonopericardioducts (20 μm in diameter), opening to a short globular pericardium (75 to 80 μm long, 67.5 μm wide, 35 to 40 μm high; Figure 5 View FIGURE 5 Q-6). The pericardioducts (65 μm long, 12.5 to 25 μm in diameter) are perpendicular to the pericardium and emerge dorsally into the spawning ducts. Around the middle of each of the pericardioducts is a sac-like structure (20 to 25 μm long, 35 to 50 μm wide, 50 to 75 μm high) that is interpreted as a seminal receptacle ( Figure 5 View FIGURE 5 R-6).
The paired region of the spawning ducts (10 to 15 μm in length) is restricted only to its origin. Almost the entire spawning duct consists of a single, fused tube (300 μm in length). The glandular layer is formed by two cell types: in the inner region, cells are deep red and with strongly stained nuclei; and in the outer layer they are more dispersed, and the staining is lighter. Towards the middle region of the spawning duct the glandular layer is thicker (92.5 to 94 μm width) and the lumen of the duct is smaller (40 to 75 μm wide, 7.5 to 12.5 μm high) than in the posterior region (107.5 μm wide, 32.5 μm high). The spawning duct discharges into the center of the mantle cavity above the copula-tory stylets as a wide duct (50 to 57.5 μm in diameter) surrounded by a thin layer of longitudinal musculature (2.5 μm thick) and the glandular layer is reduced (10 to 12.5 μm in thickness). In addition, and as already noted, there is an internal ventral pocket in the mantle cavity with glandular walls and in which there are remains of the insertion of a pair of simple short copulatory stylets.
Remarks on the genus Nematomenia . The three species described here are placed unequivocally within Dondersiidae as they all have at least two types of scales, a non-serrated monoserial radula, and type A ventrolateral foregut glands. The distinction between the different genera of the family is based on a combination of internal characteristics of which the radula is particularly informative ( Table 3 View TABLE 3 ). Of the eleven known species of Nematomenia , only two ( N. ptyalosa Salvini-Plawen, 1978 and N. tegulata Salvini-Plawen, 1978 ) have a radula. The characteristic radula in Nematomenia is a monoserial radula with four straight denticles per tooth where the neighboring denticles on either side of the radula are joined at the apex. Thus, N. divae sp. n. was easily classified. For the other two species (N.? guineana sp. n. and N. brasiliensis sp. n.), it was not possible to describe a complete radula. Nevertheless, it can be safely stated that it is a monoserial radula with at least two denticles per tooth.
The appearance of the base of the tooth and the fragments of the denticles of N.? guineana agrees with the drawings of the two Antarctic Nematomenia species described by Salvini-Plawen (1978). This does not rule out other genera. However, the habitus and sclerites of N.? guineana sp. n. do not coincide with Stylomenia or Ichthyomenia ( Thiele 1913c; Schwabl 1955; Salvini-Plawen 1972, 2003a; Handl et al. 2001; García-Álvarez et al., 2014). In addition, N.? guineana sp. n. lacks a posterior lobe or projection and copulatory stylets, making it different from Dondersia or Lyratoherpia ( Scheltema et al. 2012) , but the posterior region was not reconstructed for the new species and more data are needed. More information is also needed to rule out Micromenia , although in this genus all but one species have a separate atrium and mouth, and the presence of a dorsoterminal sensory organ is common ( Cobo & Kocot 2020). In addition to a radula that resembles the radulae known from other Nematomenia species, N.? guineana sp. n. does not have intestinal constrictions, respiratory folds, or copulatory stylets but has a common atrio-buccal cavity, all of which are traditionally considered diagnostic for Nematomenia ( Simroth 1893; García-Álvarez & Salvini-Plawen 2007). In this regard, the classification of N. brasiliensis sp. n. was somewhat challenging as it has copulatory stylets. However, it is important to note that, of the eleven known species of Nematomenia , the characteristics of the posterior region are known only for five of them: N. flavens ( Pruvot, 1890) ; N. banyulensis ( Pruvot, 1890) ; N. platypoda ( Heath, 1911) ; N. glacialis Thiele, 1913a ; N. incirrata Salvini-Plawen, 1978 ; and N. tegulata Salvini-Plawen, 1978 . In contrast, the inclusion of N. brasiliensis sp. n. in the genus can be justified according to some clear similarities with three Nematomenia species including the type species ( N. banyulensis , N. flavens and N. ptyalosa ): a) The habitus of N. brasiliensis sp. n.: a long slender animal, slightly lateral compressed with glassy scales (scaly appearance) and with dorsal keels/ridges ( Pruvot 1890; Heath 1911; Salvini-Plawen 1978). b) The helicoidal shape in which the new species is fixed suggests that it is an epizooic animal like the aforementioned known species. c) The existence of a dorsal foregut gland. d) The fact that the ventrolateral foregut gland ducts fused before opening into the foregut, which was also considered typical of Nematomenia ( Handl & Todt 2005) . e) Although the radula is ambiguous, as the arrangement of the denticles is not known, the base of the teeth and elongate denticles are similar in shape to those of N. divae sp n. f) Sclerites of N. brasiliensis sp. n. differ markedly from sclerites of the other species of Nematomenia , which provides evidence that this is a different species, but the fact that there are many different types of sclerites is shared by other species of Nemtaomenia such as N. banyulensis or N. arctica Thiele, 1913b .
The three new species have a scleritome clearly different from that of the other species of the genus Nematomenia and even from that of the all other species of the family. In addition, other anatomical features clearly differentiate them from the known Nematomenia species with radula. Nematomenia divae sp. n. and N. brasiliensis sp. n. have leaf-shaped and oar-shaped (=pallet-shaped) scales similar to those of N. ptyalosa and N. tegulata (SalviniPlawen, 1978), whereas N.? guineana sp. n. has leaf-shaped and laminar scales. The sclerites of N. brasiliensis sp. n. are even more characteristic, distinguishing this species from all other Nematomenia species.
Another important difference between species is related to the ventrolateral foregut glands (Type A sensu Salvini-Plawen 1978). In N. divae sp. n., N. brasiliensis sp. n., N. ptyalosa and N. tegulata these glands have simple, short and wide ducts (blister-shaped). In the new species, the extraepithelial glandular cells open into the ducts mostly in their anterior region, with the posterior part of the ducts with inner musculature but almost lacking glandular cells. As these glandular cells were not observed to have posteriorly bent necks, we classified them here as Pararrhopalia - type. However, unlike Pararrhopalia , the glandular cells are restricted to the anterior region of the ducts and not running along the entire extension of the organs. The lack of glandular cells in the posterior part of the duct was described as characteristic of Helluoherpia ( Handl & Todt 2005) in which, however, the ducts are long. The diminution of the ventrolateral foregut gland size is correlated with a tendency towards reduction of the radula ( Salvini-Plawen 1978; Handl & Todt 2005). In N.? guineana sp. n. the ducts of the gland are long with glandular cells opening into the tubes along the their entire extension and posteriorly, bent necks of the glandular cells were observed ( Acanthomenia - type sensu Handl and Todt 2005). According to the review carried out by Handl and Todt (2005), in Nematomenia the ducts of the ventrolateral foregut glands open unpaired and ventrally through a papilla into the foregut. Of the species described here, this occurs in N. divae sp. n. and N.? guineana sp. n., while in N. brasiliensis sp. n., they connect with the foregut as two independent ducts with one on either side of the foregut. We consider that a review of this important character is needed in order to determine if there are interspecific differences within a genus or if, in combination with other characteristics, could be or not considered as diagnostic.
The descriptions of the new Nematomenia species provide important information about the range of anatomical characteristics and distribution of this genus. Detailed re-descriptions of all species Nematomenia would be desirable to accurately determine the diagnostic characters of this genus and confirm the assignment of N.? guineana sp. n. The designation of many species of this genus is based mainly on the sclerites ( Salvini-Plawen 1978), which have a clear pattern (leaf-like scales as major sclerites with pallet-shaped or laminar scales scattered between them). However, this pattern is similar to those of Micromenia , Dondersia and Lyratoherpia . In addition, there is a lack of information on some of the genus-level diagnostic characters for many species of Nematomenia (especially of the posterior part of the body) because the details were not mentioned in the descriptions or information about the posterior part of the body is missing altogether. One character that may generate some confusion is the presence of a dorsoterminal sensory organ. The presence of this organ was described in three known species: N. banyulensis , N. incirrata and N. glacialis ( Pruvot 1890; Thiele 1913a,b,c; Salvini-Plawen 1978; Handl et al. 2001) and in N. brasiliensis sp. n., but it is absent in N. platypoda and N. tegulata ( Heath 1911; Salvini-Plawen 1978). In addition, it has not been reported for the remaining species of this genus ( N. corallophila , N. flavens , N. artica , N. protecta , N. squamosa , N. ptyalosa ), however it is not possible to state whether it exists or not, since the information on the characters of the posterior region of these species is incomplete or unknown ( Pruvot 1890; Thiele 1913; SalviniPlawen 1978). Therefore, the diagnostic validity of the dorsoterminal sense organ, in this case, at genus level does not exist. Nevertheless, it is included in the diagnosis provided here (“with or without dorsoterminal sensory organ”) as it is commonly reported as diagnostic at this level of classification and it is useful to be aware that it could be present. Similarly, presence/absence of copulatory stylets are included in the new diagnosis This character has not been reported previously for Nematomenia , but it is present in N. brasiliensis sp. n.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SuperClass |
Aplacophora |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |