Kamimuria peppapiggia, Mo & Yan & Wang & Li & Murányi, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4668.4.9 |
|
publication LSID |
lsid:zoobank.org:pub:3C525B8A-775A-4E1A-B66F-318439CEC554 |
|
DOI |
https://doi.org/10.5281/zenodo.5922788 |
|
persistent identifier |
https://treatment.plazi.org/id/305F87C6-8373-FFB8-FF59-8949FF41F813 |
|
treatment provided by |
Plazi |
|
scientific name |
Kamimuria peppapiggia |
| status |
sp. nov. |
Kamimuria peppapiggia View in CoL sp. n.
( Figs. 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
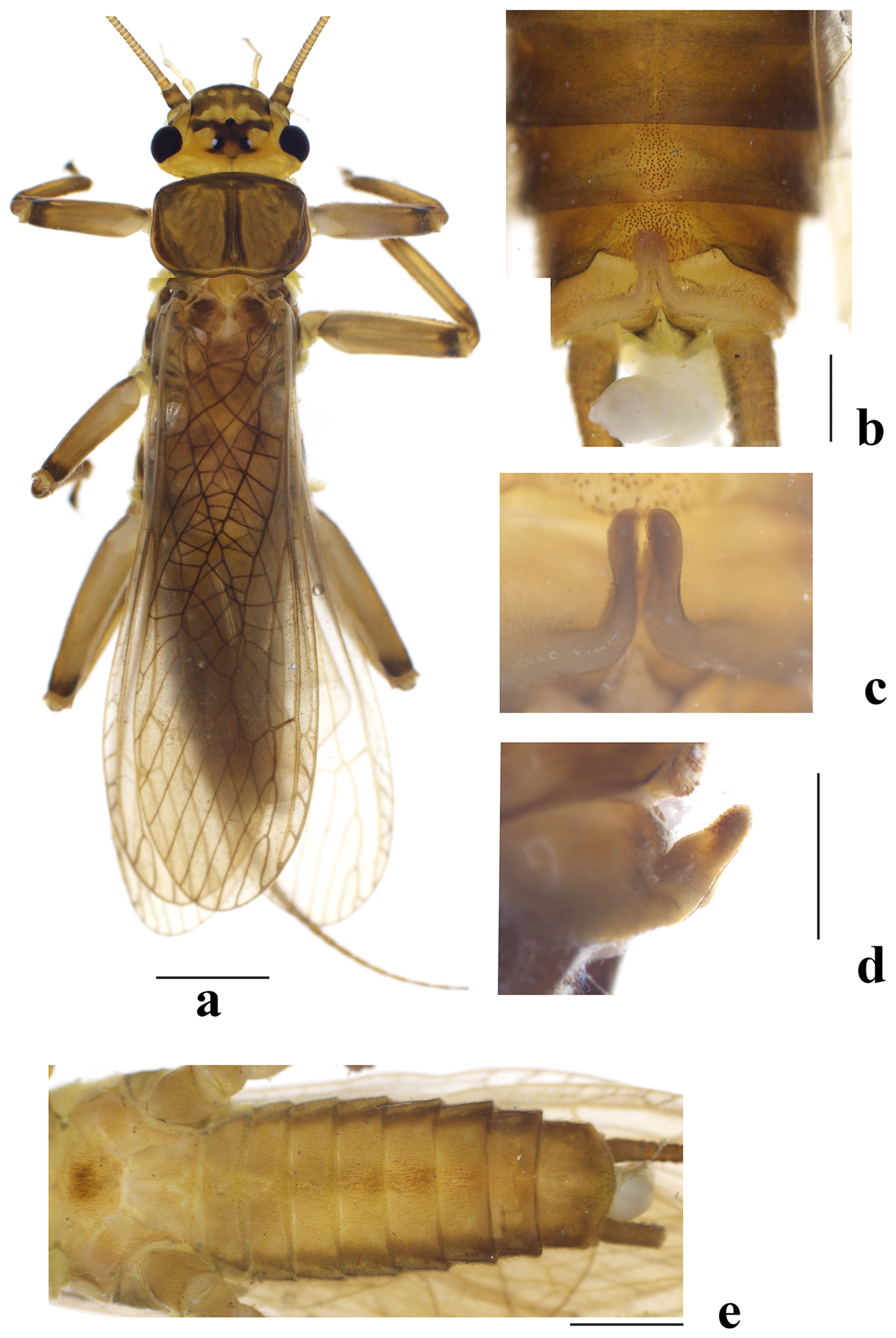
Adult habitus ( Figs. 1a View FIGURE 1 , 3a View FIGURE 3 , 8d View FIGURE 8 ). General color brownish to brown. Head yellow brown with dark pattern; black ocellar patch subquadrate, small posterolateral extensions acute and posterolateral portion less pigmented, anterolateral extensions between M-line and tentorial callosities distinct and rod-like; frons anterior to M-line brownish to dark brown. Antennae yellow brown to dark brown, palpi pale. Pronotum brown with distinct rugosities, margins and strips along median suture dark brown. Legs brownish, femorotibial joint darker. Wing membrane brownish with veins brown.
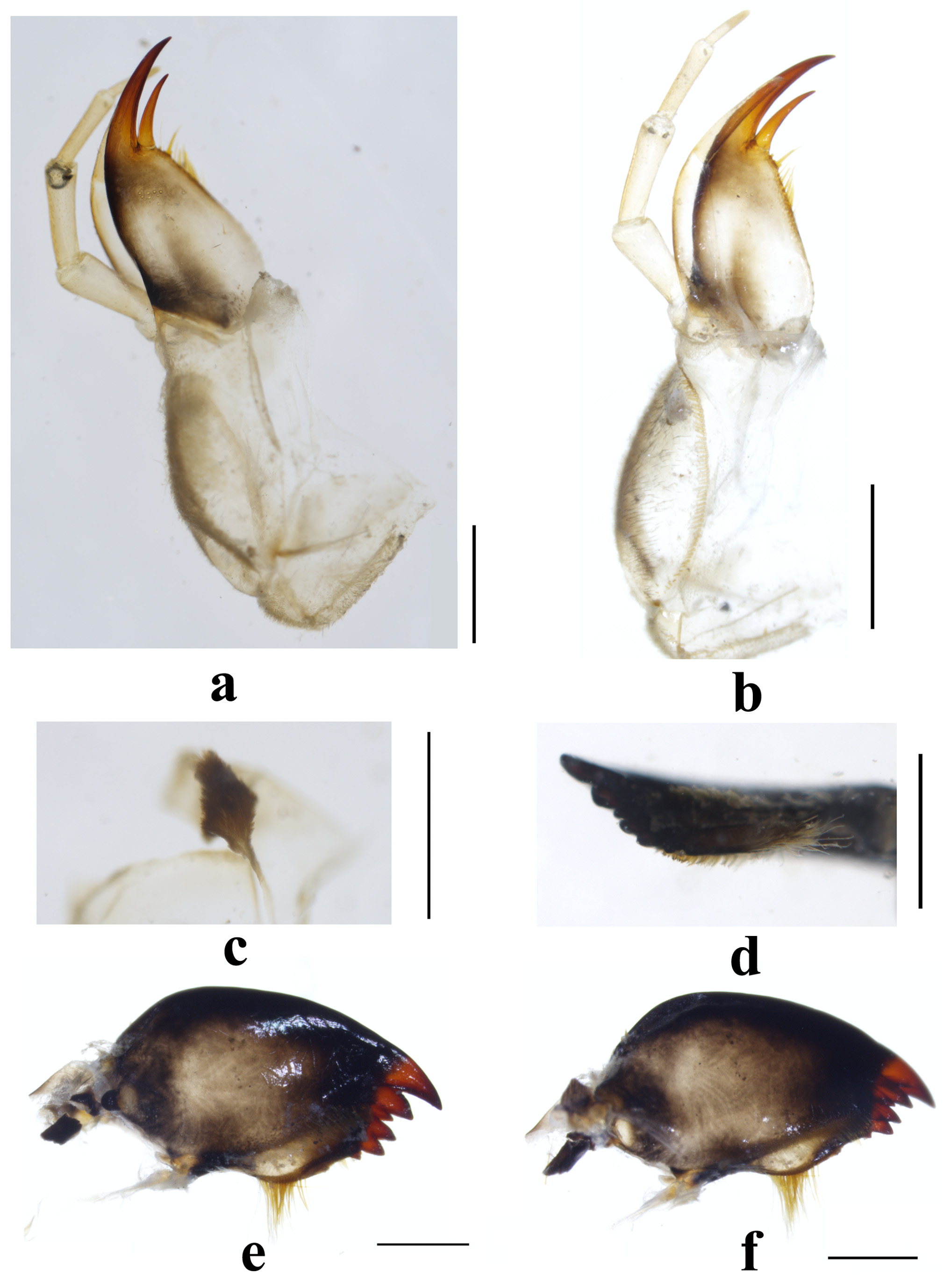
Male ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Forewing length 16.0– 16.5 mm, hindwing length 13.5–14.5 mm. Hemitergal lobe extending backward beyond posterior margin of tergum 9; the lobe in dorsal view enlarged apically while in lateral view with basal half robust and apical half narrowing and armed with ventral spinules ( Figs 1 View FIGURE 1 c–d). Tergum 9 with wide deep notch posterior to mesal sclerite, which is covered by dense sensilla basiconica; posterior margin of the sclerite produced and arched. Sensilla basiconica patch also occurs on both terga 8–9, that on tergum 8 is denser and larger than on tergum 7 ( Fig. 1b View FIGURE 1 ). Metasternum, and sterna 4–7 with setal brush ( Fig. 1e View FIGURE 1 ).
Aedeagus ( Fig. 2 View FIGURE 2 ). Basal half bald except few, indistinct spinules on the mediolateral swellings. Distal half multilobed: apex with a prominent median and a pair of ventrolateral lobe of near same size and spinulation, these lobes reminiscent of pig's head; subapical lobe consisting of a large dorsal projection and a pair of small ventral lobes; microtrichia only present on apex of these lobes. Arrangement and size of the armatures are similar on all lobes, except the denser and larger spines on the mesoapical lobe in dorsal aspect.
Female ( Figs. 3 View FIGURE 3 ). Forewing length 23.0–24.0 mm, hindwing length 22.0– 22.5 mm. Generally similar to males, abdomen of both specimens are full of mature eggs. Sterna 8–9 with elliptical mediolateral black stigma, sternum 9 medially white. Subgenital plate with two widely separated posterolateral lobes; the lobe longer than wide and apical half triangular, tip obtuse; the hollow between lobes nearly quadrate, with wider opening. Vagina subrectangular, genital opening wide, anteromedian portion sclerotized and wrinkled. Spermathecal stalk slender, about 2X longer than vagina. Spermatheca ovum-shaped, with longitudinal wrinkles.
Egg ( Fig. 7 View FIGURE 7 ). Length 0.42–0.54 mm, width 0.26–0.34 mm (N=6). Outline oval shape with obscure opercular line, widest in subequatorial area towards opercular end. Anchor mushroom-shaped, with globular bodies evenly distributed and not grouped. Collar short but distinctly flanged, with heavy meshwork of irregular shaped follicle cell impressions (FCIs) towards collar rim. Chorionic surface with shallow and indistinct punctations throughout, small and white globular bodies widely distributed, FCIs recognizable only on collar end. Micropylar orifices small and mostly with rim, much larger than punctations, set on subequatorial line.
Larva ( Figs. 4– 6 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 8e View FIGURE 8 ). Body length of the pharate female larva 21.6 mm, ultimate male larva 12.4 mm, first year larva 8 mm, male exuviae 23.0– 23.5 mm, female exuviae 25.0 mm; no last instar male larva available. General color brown to dark brown with distinct and symmetrical dark markings on dorsal surface ( Fig. 4 View FIGURE 4 ); ventral surface generally paler.
Mature larva: Head brown with symmetrical dark brown markings from occipital area to M-line, frontoclypeal area darker, with two distinct yellow brown patches surrounding two posterior ocelli. A pair of tentorial callosities rod-like, about twice as long as diameter of a posterior ocellus. Triocellate, anterior ocellus smaller; distance between ocelli about twice as wide as diameter of one posterior ocellus. Occipital ridge and occipital suture distinct and bearing row of stout occipital setae. Antenna mostly yellowish brown, scape, pedicel and base of flagellum darker. Lacinia bidentate, apical tooth much longer than subapical tooth, marginal fringe with row of setae; galea shorter than apical tooth of lacinia, armed with several setae; stipe ventral surface with a larger patch of setae and a few scattered punctations ( Figs. 6 View FIGURE 6 a–6b). Mandible wide, having three molar and two incisor dens, molar brush short but dense ( Figs. 6 View FIGURE 6 d–6f). Hypopharynx brown, with short dense apical setae ( Fig. 6c View FIGURE 6 ). Pronotum rectangular with rounded corners and lateral margins, length about twice as long as width, marginal row of setae laterally incomplete; all thoracic segments covered only by fine, black clothing hairs, and dark brown coloration is covered in most areas ( Fig. 4 View FIGURE 4 ). Wingpad dark brown, covered with black scattered setae; two rows of long, black, sparse, mesal hairs arranged along ecdysial suture, more pronounced in younger larvae ( Fig. 4c View FIGURE 4 ). Proventricular teeth uniform and regularly placed ( Fig. 5c View FIGURE 5 ). Legs relatively long, swimming hairs long and dense; surface setation of femore consist of black clothing hairs and brown, thick setae, but tibial setation is scarce and only having rows of setae on margins ( Fig. 5b View FIGURE 5 ). Abdomen with terga 1–9 mostly dark, while tergum 10 with paler medial and posterolateral strips ( Figs. 4 View FIGURE 4 a–4b). All terga bear dense apical row of long setae, that are brown and easily broken; similar setae are placed in an irregular, transverse median row on all terga, while most of the surface is covered with fine, black clothing hairs ( Figs. 4a View FIGURE 4 , 5e View FIGURE 5 ). Basal sterna are brown but sterna 7–10 are mostly paler, with thin clothing hairs, female sternum 8 is narrowly interrupted by shallow and triangular genital notch ( Fig. 5a View FIGURE 5 ); posterior row of setae complete only on sterna 9–10. Paraproct simple, without anal gill ( Fig. 5a View FIGURE 5 ). Cercus long, with long and dense swimming hairs are forming distinct tufts on the inner surface of cercomeres; further portions of the row consist of short but not erect, brown setae mixed with short, fine hairs ( Fig. 5d View FIGURE 5 ).
Type material. Holotype male ( HIST): China: Henan Province, Weihui City, Xiaodianhe Village , small and slow flowing river, 35°36.747’ N, 114°00.289’ E, 210 m, 29.iii.2019, leg. Fanbin Kong, WeiHai Li, RaoRao Mo, Dávid Murányi. GoogleMaps Paratypes: same locality and date as holotype: 1 female, 1 ultimate male larva, 1 pharate female larva, 2 first year larvae, 1 male exuviae, 2 female exuviae ( HIST) GoogleMaps , 1 male, 1 female, 1 ultimate female larva, 1 male exuviae ( HNHM) ; same locality as holotype, 16.v.2012, collected by undergraduate students: 1 female ( HIST) GoogleMaps .
Etymology. The specific epithet comes from the popular cartoon figure Peppa Pig, referring to the pig-like shaped aedeagal sac. The Chinese name of ‘peppa’ also means ‘armed with everything needed or complete equipment’, implying that the new species is known from all life stages, and caught in the year of the pig.
Remarks. Males of the new species are most similar to K. manchuriana Wu, 1938 from northeastern China by having a similar multilobed aedeagal sac and hemitergal lobes in lateral view ( Sivec & Stark 2008). However, it may be easily separated from K. manchuriana by having large sensilla basiconica patches on terga 8–9, which are found only on tergum 9 and the posterior edge of tergum 8 in case of K. manchuriana . The females of K. peppapiggia are distinguished from all other Kamimuria species by its unique prolonged posteriolateral lobes of subgenital plate. The egg is most similar to that of K. fulvescens Klapálek, 1912 , but differs by less pronounced FCIs of the chorion. The larva can be distinguished from the few known larvae of congeners based on its distinctive head pattern.
Distribution and ecology. The new species was found in a single river flowing through the foothills of the Taihang Mountains in Henan Province of China. The entire length of the river is about 20 kilometers together with its longest source tributary, interrupted by three large and two smaller reservoirs. The type locality is about two kilometers upstream to the lowest reservoir, and five kilometers far from the site where the river reaches the North China Plain and disappears from its original riverbed, into diversion canals. The foothill range where the watershed reaches 1000 meters at its highest ridge and expands eastward into the North China Plain from the main massif of the Taihang Mountains.
The river is about 15 meters wide at the type locality, having a stony substrate and decreased flow ( Figs. 8 View FIGURE 8 b–c). The river depth varies between half to two meters, with dense submerged vegetation but covered by filamentous algae at several, mostly littoral sections. The riparian vegetation includes poplars, willows, reedy or ruderal vegetation. All Kamimuria specimens were found in one of two artificial rapids crossing the whole section of the waterflow ( Fig. 8a View FIGURE 8 ). Both larvae and adults were hiding beneath partly submerged stones and occurred in low numbers. The new Kamimuria was the only stonefly found, besides a few Ephemeroptera, Trichoptera, and several Diptera species. Many small fish were observed frequently jumping out of water and feeding on flying Diptera and Trichoptera. The water was relatively warm t herefore, the river seems suitable only for potamal stoneflies. The species has an early emergence period, starting in early spring and lasting at least middle of May. Given its special habitat and its absence from the higher streams and rivers of the Taihang Mountains that have been frequently sampled by us, it can be presumed that the new species is a remnant of the lowland stonefly fauna that has been probably extirpated most of in the North China Plain. Kamimuria peppapiggia may be the only survivor in the rapids of this foothill river.
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |