Microhyla nepenthicola, Das, Indraneil & Haas, Alexander, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.197388 |
|
DOI |
https://doi.org/10.5281/zenodo.6196484 |
|
persistent identifier |
https://treatment.plazi.org/id/30618275-FFD0-8E6A-FF75-AADCFF4BF837 |
|
treatment provided by |
Plazi |
|
scientific name |
Microhyla nepenthicola |
| status |
sp. nov. |
Microhyla nepenthicola View in CoL sp. nov.
( Figs. 2–4 View FIGURE 2 View FIGURE 3. A View FIGURE 4 )
Holotype: ZRC A.12431, adult male, from near 1,000 feet marker, Summit Trail, Gunung Serapi (01º36’24”N, 110º11’24”E [WGS84]), Kubah National Park, Matang Range, Sarawak, East Malaysia (Borneo); altitude ca. 300 m asl. I. Das and A. Haas, 4 September 2004.
Paratypes: ZRC A.12432-34, paratopotypes, other data as for holotype, adult males; ZRC A.12435-36, paratopotypes, adult females, I. Das and A. Jankowski, respectively, 11 May 2005 and 29 October 2005, respectively; ZRC A.12437–40; paratopotype, adult males other data as for holotype, A. Jankowski, 13 September 2005, 29 November 2005, 7 December 2005 and 28 January 2006, respectively.
Diagnosis: We allocate these specimens to Microhyla for showing the following characters diagnostic for the genus ( Parker 1934; Inger 1966; Malkmus et al. 2002): narrow head; body flattened; eyes reduced; maxillary and vomerine teeth absent; reduction of Finger I; toes with reduced webbing; pupil circular; tongue large, oval and entire; an inner metatarsal tubercle under each foot; larvae lacking keratinized beaks; terminal mouth and laterally-positioned eyes and vent embedded medially in lower tail fin. A small (SVL 10.6–12.8 mm in eight males; SVL 17.9 and 18.8 mm in the two females) species of Microhyla , diagnosable from congeneric species in showing the following combination of characters: dorsum with low tubercles that are relatively more distinct on flanks; a weak, broken, mid-vertebral ridge, starting from forehead and continuing along body; no dermal fold across forehead; tympanic membrane and tympanic annulus absent; Finger I reduced to a nub proximal to Finger II in males; toe tips weakly dilated; phalanges with longitudinal grooves, forming two scale-like structures; webbing on toe IV basal; toes with narrow dermal fringes; inner and outer metatarsal tubercles present; and dorsum brown with an hour-glass shaped mark on scapular region.
Description of holotype: Adult male, SVL 11.8 mm; body subtriangular, depressed; body width 6.0 mm; axilla to groin distance 4.8 mm; head length 3.4 mm; head width 3.4 mm; head as wide as long (HW/HL ratio 1.00); head depth 2.7 mm; snout obtusely pointed when viewed dorsally and especially laterally; projecting well beyond mandible, but not tapering in dorsal view; nostrils laterally positioned, nearer tip of snout than to eye, eye to snout distance 1.7 mm; eye to nostril distance 1.1 mm (E-N/E-S ratio 0.64); internarial distance (1.4 mm) greater than distance from anterior margin of eye to nostril (IN/E-N ratio 1.27); eye small (diameter 1.3 mm); smaller than half of head length (ED/HL ratio 0.39); its diameter slightly greater than eye to nostril distance (ED/E-N ratio 1.18); interorbital width (2.7 mm) greater than upper eyelid width (1.0 mm) (IO/UE ratio 2.70); canthus rostralis obtuse; loreal region vertical; a weak ‘W’- shaped notch (= symphysial knob) on anterior edge of mandible; mouth extends to posterior corner of eye; choanae located against anterior of palate visible when viewed from below; teeth absent on maxilla and vomerine regions; no dermal ridges across palate; tongue oval, smooth, rounded apically, free for approximately half its length; pupil rounded; tympanic membrane and tympanic annulus not visible externally; weak fold across supratympanic region extends from posterior corner of orbit to before insertion of forelimbs; cloacal opening at mid-level; median subgular vocal sac.
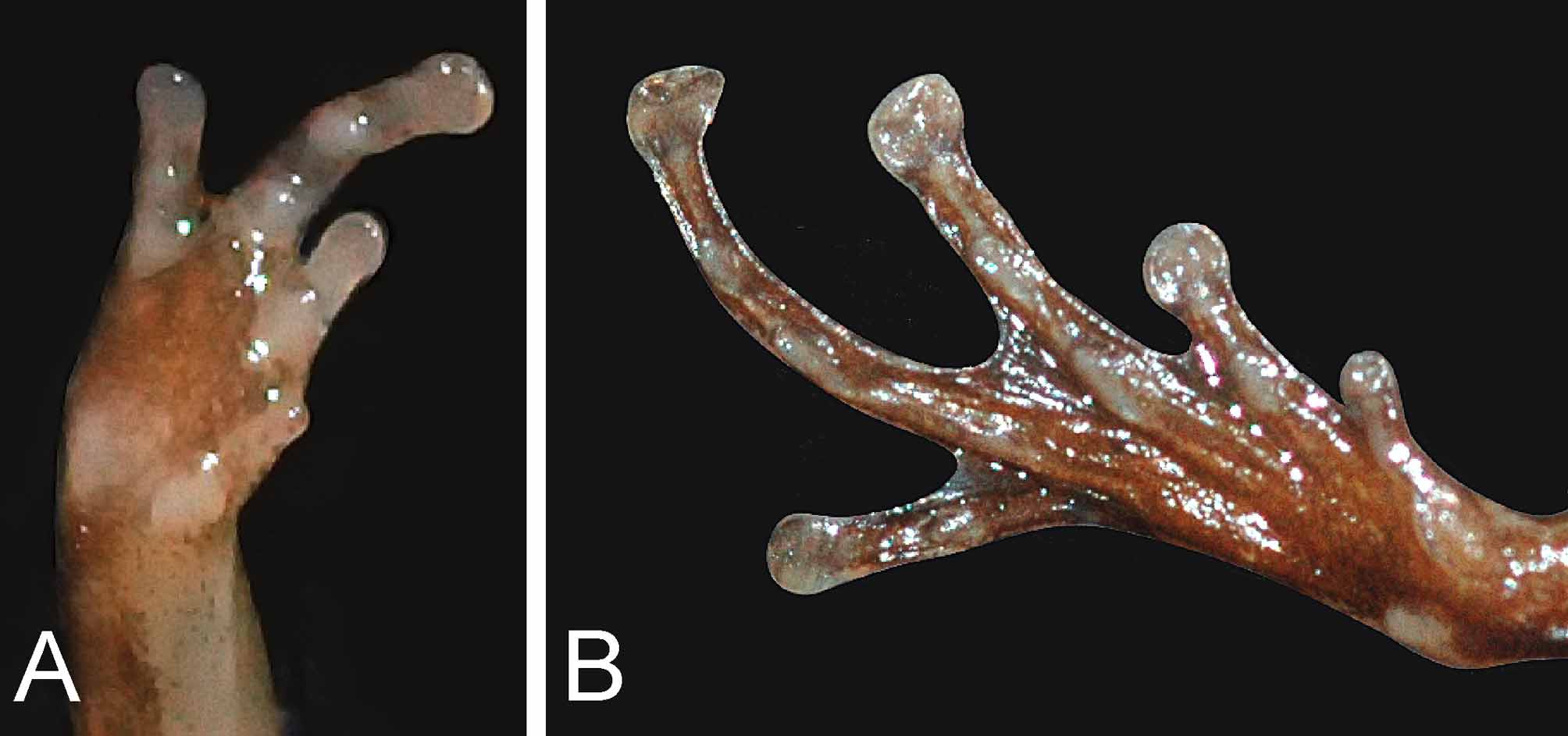
Fore limbs short; fingers free of web or skin fringes; relative length of fingers (measurements in parentheses, in mm): 3 (1.6)> 2 (1.1)> 4 (0.7)> 1 (0.4); finger tips weakly swollen but not dilated, lacking dermal fringe; dorsal surface of all fingers with longitudinal grooves forming a two-scaled structure; Finger I reduced to a nub or a tuberculate structure, located proximally to Finger II; subarticular tubercles prominent on Fingers II–IV (absent on Finger I), rounded, numbering one on second finger, two on third and fourth fingers; fleshy palmar tubercles; nuptial pads absent on fingers; no enlarged glands on lower arm.
Hind limbs short; tibia length 7.5 mm; dorsal surfaces of thigh and tibia nearly smooth; dorsal surface of all toes with longitudinal grooves forming a two-scaled structure; toes basally webbed- webbing on Toe 1 basal; on Toe II basal (inner) and level between subarticular tubercle and distal swelling (outer); Toe III basal subarticular tubercles (inner) and distal subarticular tubercle (outer); Toe IV basal subarticular tubercle (inner); relative length of toes (measurements in parentheses, in mm): 4 (5.1)> 3 (3.5)> 2 (2.2)> 5 (1.7)> 1 (0.5); toe tip weakly dilated; subarticular tubercles prominent, rounded, numbering one on first and second toes; two on third and fourth toes; a large, pale, elongate compressed outer metatarsal tubercle, that is not shovel-shaped, and a slightly smaller, darker, inner metatarsal tubercle.
Dorsum with low tubercles that are relatively more distinct on flanks; a weak midvertebral dermal fold that extends from postorbital region to above vent; eyelids and upper surfaces of limbs smooth; a weak and narrow supratympanic fold extends from posterior corner of orbit of eye to axilla; abdomen and inner side of thighs smooth.
Colour: In life, dorsum Orange-Rufous (Color #132C), with scattered Peach Red (Color #94) patches on upper surfaces of body and limbs; snout region Vinaceous (Color #3); a Raw Umber (Color #223) hour-glass pattern with a somewhat irregular outline, whose anterior edge lies across interorbital region, covering the anterior third of dorsum, its edges most distinctly indicated; Robin Rufous (Color #340) paired subtriangular patches on either sides of midbody; snout-tip with two coalesced Sepia (Color #119) ovoid blotches; upper lips with two Sepia (Color #119) patches; a Pale Horn (Color #92) stripe, half eye diameter in thickness, runs obliquely on temporal region, corresponding to the dermal fold behind the angle of the jaws; a Jet Black (Color #89) stripe, with a Peach Red (Color #94) dorsal edge, on scapular region, that extends from level of junction of fore arm, and curves slightly ventrally, terminating past midlevel of body, the dorsal border of stripe is well defined, ventral border wavy and interrupted; ground colour of flanks slightly paler than that of dorsum; thigh, shank and lower arm Orange-Rufous (Color #132C), with narrow Dark Brownish Olive (Color #129) bands; phalange similarly coloured; anterior edge of thighs with an elongated Sepia (Color #119) patch close to the level of the knees; a short Dusky Brown (Color #19) bar across anal region; gular region Pale Pinkish Buff (Color #121D) with Walnut Brown (Color #221B) mottling; pectoral and abdominal regions unpatterned cream; undersurfaces of fore and hind limbs Raw Umber (Color #223). Pupil black, iris Buff- Yellow (Color #53), within which area extensive areas with dark reticulation; iris with a golden sheen, especially around the pupil, on each side of pupil, Salmon Color (Color #106).
Measurements (in mm): Male holotype and paratypes; n = 8, range (mean, SE): SVL 10.6–12.8 (11.7, 0.28); HL 2.3–3.9 (3.0, 0.18); HW 2.4–3.7 (3.3, 0.15); HD 2.2–2.9 (2.45, 0.09); BW 3.6–6.6 (5.41, 0.39); TBL 6.4–8.4 (7.50, 0.23); ED 1.2–1.7 (1.45, 0.06); UE 0.0–1.2 (1.03, 0.03); IN 1.1–1.5 (1.31, 0.04); IO 2.2– 2.7 (2.53, 0.06); E-S 1.5–2.2 (1.83, 0.09); E-N 1.5–2.2 (1.83, 0.09); and A-G 4.2–5.3 (4.9, 0.16); Female paratypes; n = 2: SVL 17.9 and 18.8; HL 4.6 and 4.0; HW 4.8 and 5.4; HD 3.3 and 2.6; BW 9.2 and 10.0; TBL 11.4 and 12.2; ED 1.5 and 1.7; UE 1.1 and 1.2; IN 1.8 and 1.8; IO 3.6 and 3.2; E-S 2.8 and 2.9; E-N 1.7 and 1.6; and A-G 7.8 and 7.9.
Variation: The sample reveals sexual size dimorphism in the new species, with the two females exceeding the eight males in size (U -test, p <0.05). Within the sample, the dorsal pattern was obscured or lost, or show two fused hour-glasses pattern that nearly reach the anal region. Females show greater development of Finger 1, with one phalange clearly visible. The reduction of elements on Finger I in the diminutive males is possibly an effect of a smaller body size. In preservative (70% ethanol), the dorsum is smooth, tubercles being indistinct. Additionally, the saddle-shaped mark and pale snout are noticeably less distinct.
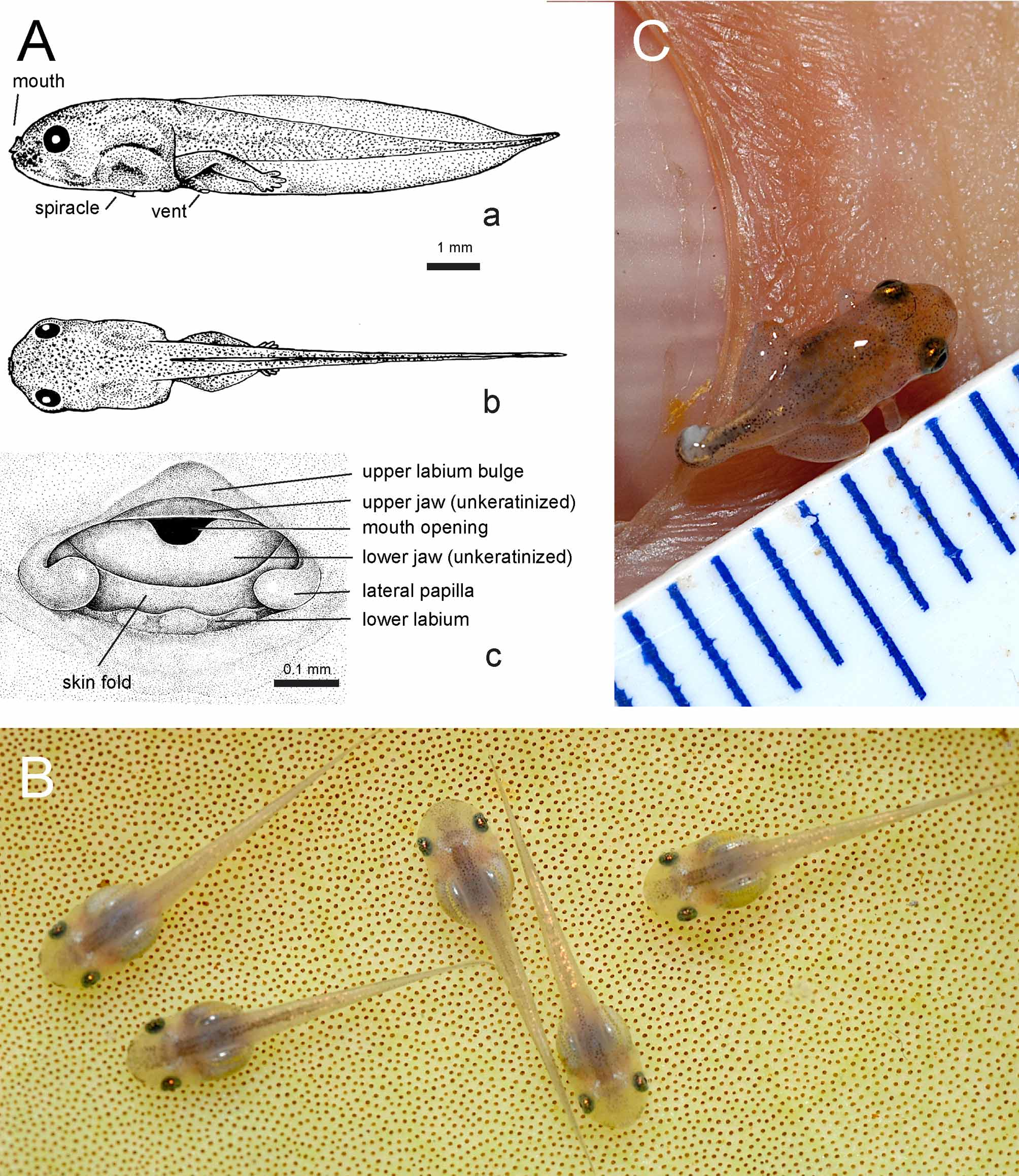
Larvae: Larval voucher specimens: ZMH A10014–10020; ZMH A10044; ZMH A10048; ZMH A10177– 78; illustration based on ZMH A 10015, stage 36. External morphological features ( Figs. 4 View FIGURE 4 Aa–b): Larvae reach a total length of 9–11.3 mm; tail length 70% and head-body length 30% of total length; body contour in dorsal view parallel sided, as broad at eye level as at mid-body level; body approximately as wide as deep; a slight constriction of body contour behind level of eye; snout short; eyes lateral in position and relatively large; no external narial opening in pre-metamorphic stages; spiracle lies ventrally in mid-sagittal plane, approximately in mid-belly region ( Fig. 4 View FIGURE 4 Aa); spiracular orifice is a caudad directed opening with a straight but slightly irregular rim (ventral view); gut arranged in a short coil, often with only three bends in lateral view; gut visible through the skin; lungs functional, serving as buoyancy organs, and shine through dorsal skin as silvery, dorsal trunk structures.
Tail fins start at trunk-tail junction ( Fig. 4 View FIGURE 4 Ab); ventral tail fin higher than dorsal tail fin; tail fin edges of upper and lower fin approximately parallel in orientation, except at distal quarter of tail, where they converge with convex outlines towards tip; shortly before tip, tail edges become convex, forming a short acuminate to flagellar tip; vent embedded and opening medially in lower tail fin.
Due to the transparent appearance, lateral line neuromasts are not readily visible in living specimens. Oral orifice terminal on snout and directed forward; oral disk highly reduced: upper jaw with very shallow lip flap (bulge; Fig4 View FIGURE 4 Ac); flap on lower jaw much reduced but present and separated from a chin-like bulge below by a fold; two knob-like papillae form the corners of lower lip; between these papillae, the lower labium is highly reduced and has a undulating contour indicating three vestigial papillae; labial ridges and keratodonts absent; jaws without keratinized beaks.
Larvae show scattered pigment cells, making them appear moderately dark color when viewed with the naked eye. Under magnification, flanks, dorsum, dorsal head, and sides of tail show scattered melanocytes. Epidermal melanocytes small and irregular, often stelliform in general shape, fringed with irregular cytoplasmatic processes. Dorsum and forehead with a slight greenish hue underlying scattered melanocytes; ventral and lower lateral sides of head, and lateral gill region unpigmented; yellowish gut coils visible through abominal wall in ventral and lateral views; iris dusted with dense bronze pigmentation on a black background; scleral part of eyeball covered with reflecting iridocytes, and depending on direction and intensity of light, iridocytes may appear silvery to golden; iridocytes also present along dorsal edge of muscular part of tail in mid-tail region; iridocyte and melanocyte densities on tail low and tail myosepta visible; tail fins clear. Metamorphs average 3.5 mm, and are near translucent under natural light, when viewed by the naked eye, and digital images reveal a pale pink dorsum.
Color in preservation is different from that in life: silvery iridocytes are not visible in preservation and melanocytes bleach easily. Preserved tadpoles are thus mainly cream-colored, with translucent components (such as the tail fins and belly). The iris and sclera are black.
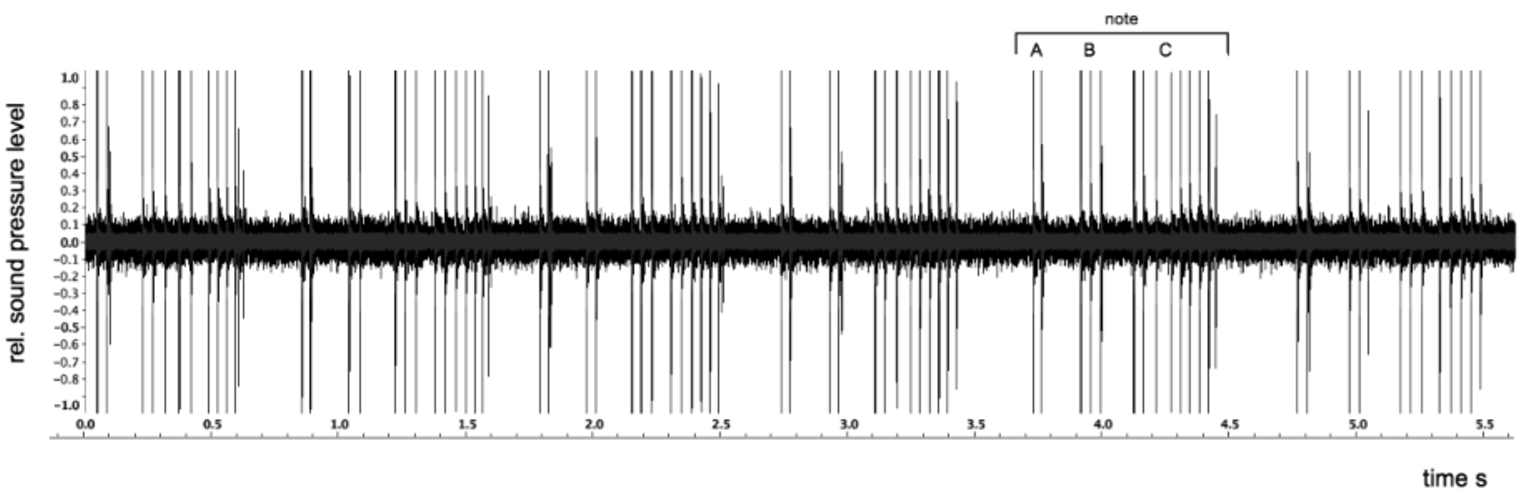
Call. Males of the new species commence calling at dusk, choruses peaking during the early hours of the evening (ca. 1845–2100 h). Males form calling aggregations within and around patches of pitcher plants, Nepenthes ampullaria . A few minutes of intense group choruses may be followed by periods of silence, or with only occasional single males calling briefly. The call can be described as a series of harsh rasping notes. The structure of a call is depicted in Fig. 5 View FIGURE 5 . Parameters of the shown call are: in aroused males, the call consists of a rapid sequence of notes. A note consists of two short (1–2) and one larger (5–9) pulse groups ( Fig. 5 View FIGURE 5 : A, B, C, respectively). The pause between pulse groups within a note was 125–154 ms; breaks between notes were 192–330 ms. Repetition frequency of notes (i.e., duration of pauses in a series) depends on the level of agitation in a calling congregation. Notes have a duration of 696–736 ms. Spectrum analysis of the notes in Fig. 5 View FIGURE 5 show that frequencies in the range of 3,000–5,500 Hz contribute most to the measured sound pressure level.
Etymology: Latin for inhabitant of Nepenthes , or pitcher plants ( Fig. 6 View FIGURE 6 ). Used as a noun in apposition.
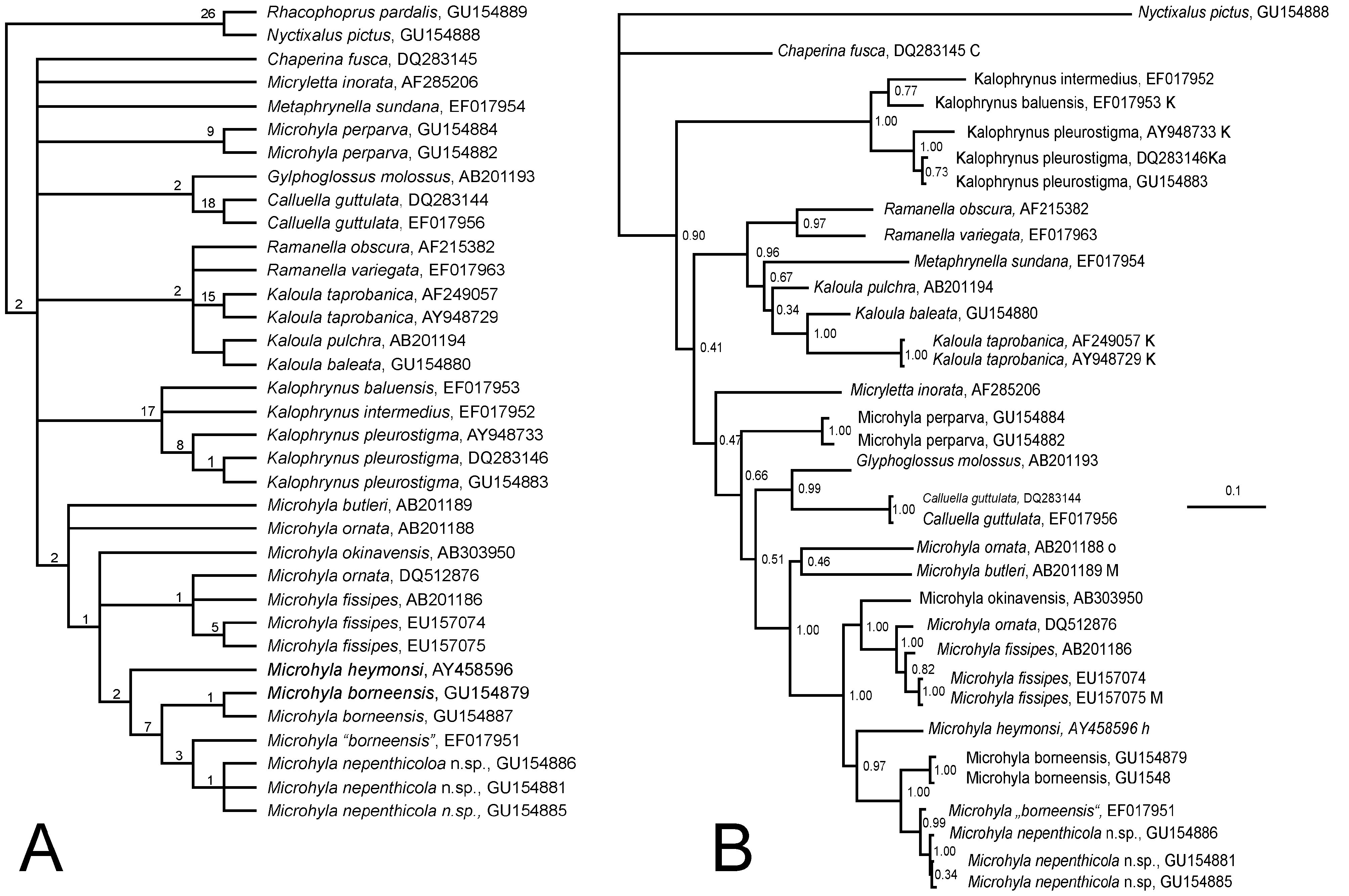
Comparisons: The preliminary phylogenetic analysis ( Fig. 7A View FIGURE 7. A ) shows that the sympatric Microhyla borneensis Parker 1928 shares a sister-relationship with M. nepenthicola sp. nov., but is clearly separated genetically by a divergence of 5.1% (syntopic ZRC 1.11939 [ GU154887 View Materials ] and ZRC 1.12432 [ GU154885 View Materials ]). Females of the new species are indeed rather similar morphologically to the more widespread M. borneensis (which is not reproductively syntopic at the type locality of the new species, breeding in large, permanent bodies of water, such as ponds). The latter species, however, is substantially larger, borneensis males reaching 18 mm (vs. 12.8 mm), females 23 mm (vs. 18.8 mm), and are further separated by the possession of a weak supratympanic fold (vs. absent); Finger I reduced, but projecting from margin of carpal margin (vs. reduced to a tubercle); tubercles forming a low ridge on flanks (vs. absent) and a light-edge to the dark pattern on dorsum (vs. absent). The larval stages of the two species are also substantially different, tadpoles of M. borneensis attaining 22.4 mm (as opposed to 11.3 mm in the new species),
The new species from Kubah is compared here with all congeners, plus the sister lineage, Micryletta , considered by some authors to be synonymous with, or a subgenus of Microhyla ( Frost 2009) . The new species differs from Microhyla , maculifera Inger 1989 , in showing a distinct outer metatarsal tubercle, dorsal grooves on toes, and first finger reduced to a tuberculate structure in males (vs. half length of Finger IV); from M. berdmorei (Blyth 1856) , M. perparva Inger and Frogner 1979 , and M. petrigena Inger and Frogner 1979 , by reduced webbing on toes that fail to reach the disks on Toes III and V. Among the remaining species of the genus, the following lack the median longitudinal grooves on disks: Microhyla chakrapanii Pillai 1977 ; M. fissipes Boulenger 1884 ; M. fowleri Taylor 1934 ; M. mixtura Liu and Hu in Hu, Zhao, and Liu 1966; M. okinavensis Stejneger 1901 ; M. ornata (Duméril and Bibron 1841) ; M. palmipes Boulenger 1897 ; M. picta Schenkel 1901 ; M. pulchra (Hallowell 1861) ; M. superciliaris Parker 1928 , and M. zeylanica Parker and Osman-Hill 1949 . Broad webbing to disks of toes separate the following congeners from the new species: M. annamensis Smith 1923 ; M. marmorata Bain and Nguyen 2004 ; M. nanapollexa Bain and Nguyen 2004 ; and M. pulverata Bain and Nguyen 2004 . Lack of webbing and of median grooves on toes separates Micryletta inornata (Boulenger 1890) and M. stejnegeri (Boulenger 1909) , which are sometimes placed in the genus Microhyla ). Distinct disks on fingers separate M. achatina Tschudi 1838 ; M. annectens Boulenger 1900 ; M. butleri Boulenger 1900 ; M. erythropoda Tarkhnishvili 1994 ; M. fusca Andersson 1942 (only on Finger III); M. heymonsi Vogt 1911 ; M. mantheyi Das, Yaakob and Sukumaran 2007 ; and M. sholigari Dutta and Ray 2000 . Its shovel-shaped inner metatarsal tubercle separates M. rubra (Jerdon 1854) from the new species. Finally, M. karunaratnei Fernando and Siriwardhane 1996 differs from the new species in showing extensive dark patches on its venter, more extensive webbing on toes that reach antepenultimate and proximal subarticular tubercle of Toe IV; and outer metacarpal tubercle completely divided.
Preliminary hypothesis of relationships: Results of preliminary phylogenetic assessment are summarized in Figs. 7A–7 View FIGURE 7. A B. The two adult (ZRC 1.12441, GU154886 View Materials ]; ZRC 1.12432, GU154885 View Materials ) and one larval (ZMH A 10019, GU154881 View Materials ) 16S RNA gene DNA samples from the type locality form a clade. The new species is nested within a more inclusive clade comprising Microhyla borneensis and M. heymonsi as closest relatives among the known Bornean species, both in MP and BI analyses. The phylogenetic position is in accordance with the morphological evidence: the new species is most similar to Microhyla borneensis in body shape and colour pattern, albeit at significantly smaller maximum body size. The two samples of M. borneensis (ZRC 1.11939, GU154887 View Materials ; ZMH A 10027, GU154879 View Materials ) from the type locality of M. nepenthicola sp. nov. are monophyletic in the analysis and genetically distinct from the new species (5.1%). Interestingly, the 16S RNA gene sequence EF017951 View Materials obtained from GenBank and deposited as “ Microhyla borneenis ” appeared as sister-taxon to the newly described species. We contacted the collector and found out that EF017951 View Materials was from a population of very small individuals, not larger than 12 mm, from Bako National Park, ca. 25 km NE of Kubah National park. The size of the frog and the present analysis, however, suggest, that EF017951 View Materials belongs to the new species (differences with M. nepenthicola , ZMH A 10019 [ GU154881 View Materials ], in the overlapping 495 bp).
Distribution and ecological notes: The adults from the type series were collected at the verge of the road leading to the summit of Gunung Serapi, at ca. 300 m asl. The locality lies within a section of kerangas (Bornean heath) forest, and the undergrowth has patchy by dense aggregations of the pitcher plant, Nepenthes ampullaria ( Fig. 6 View FIGURE 6 ). A total of at least 56 species of anuran amphibians and two caecilians occur in the Matang Range (Das et al. 2007; Haas, unpubl., Matsui 2009), including one congener ( Microhyla borneensis ).
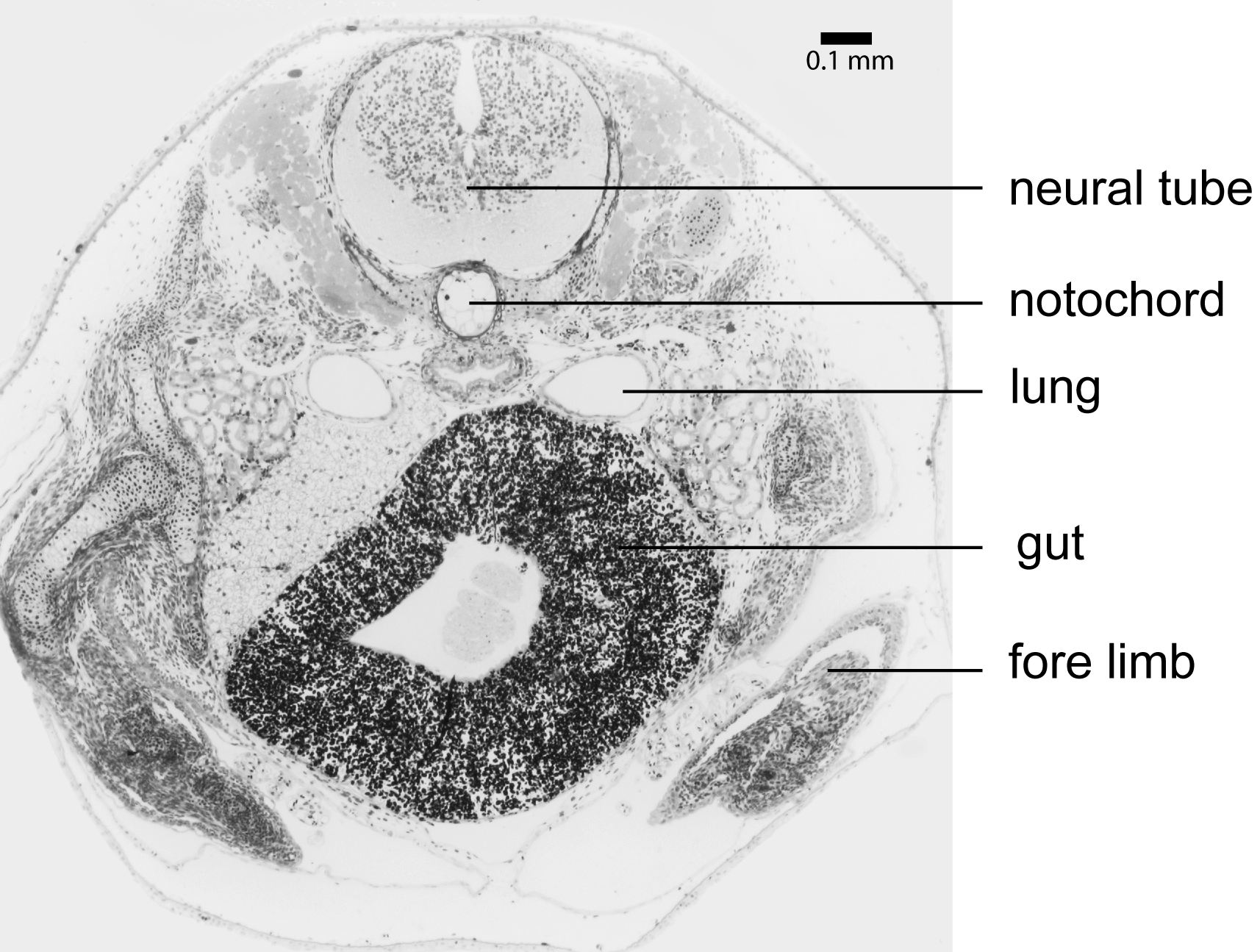
Larvae of Microhyla nepenthicola sp. nov. are endotrophic. We collected pitchers with fresh eggs, deposited on the sides of the pitchers, and observed that larval metamorphosis is completed within about two weeks after oviposition. We found up to 14 larvae in a single pitcher, showing different developmental stages, and indicating multiple egg deposition in one pitcher. In histological sections, we found large amounts of yolk platelets in the gut tissue ( Fig. 8 View FIGURE 8 ) even in specimens with advanced limb development ( Gosner 1960: Stages 28–36). The hyobranchial apparatus was reduced in all sectioned specimens (ZMH A10044; ZMH A10048; ZMH A10177–78). We collected the tadpoles from Nepenthes ampullaria pitchers next to calling males of the species. Adults and larvae were matched by DNA barcoding. Tadpoles hover in the pitcher’s liquid almost motionless; characteristically the body axis is oriented at an angle of ca. 30° with the head oriented up from level of body, and movement is facilitated by constant beating of tail tip. When disturbed, they are capable of rapid movement, trying to burrow into the debris accumulated at the bottom of pitchers. Due to their small size, pale coloration, behavioral crypsis via tendency for reduced activity, and large amount of debris (typically partially digested arthropod body parts) in the pitcher, tadpoles can be difficult to sight in situ.
Phylotelm-breeding amphibians have been reviewed by Lehtinen et al. (2004). In breeding in pitcher plants ( Nepenthes sp.), the new species is rather unusual, as only a few anurans have been recorded breeding in pitchers. Dover (1928), the first author to report breeding by anurans in Nepenthes ampullaria , identified the species as a bufonid, and possible Duttaphrynus melanostictus . Given the known size of larvae- a Stage 47 individual in the size range 7.9–9.3 mm ( Leong and Chou 1999: Table 3; see also Ye et al. 1986), it is unlikely to be this species. Other anuran amphibian species known to breed or at least reside in pitchers include both free-swimming ones, such as Kalophrynus pleurostigma (fide Lim and Ng 1991: Nepenthes ampullaria ), Kalophrynus cf. heterochirus (fide Phillipps et al. 2008: from Nepenthes stenophylla ), and those showing direct development, including Philautus aurifasciatus (fide Yong et al. 1988: from Nepenthes sanguinea ); unspecified species of Philautus (fide Kiew 1987: from Nepenthes ampullaria ; also Clarke 1997: from Nepenthes bicalcarata ; Phillipps et al. 2008: from Nepenthes hurrelliana ); Philautus mjobergi ( Smith 1925: from unspecified Nepenthes ; Philipps and Lamb 1988: from Nepenthes villosa ), Philautus kerangae ( Dring 1987: from Nepenthes bicalcarata ) and Philautus saueri ( Malkmus et al. 2002: from Nepenthes villosa ). One additional record- that of Microhyla borneensis (fide Parker 1934) is in doubt, given the known reproductive habits of this species mentioned above. We suspect Parker's (1934) material, which was taken from "Kuching" (presently ca. 30 km S of the type locality) by John Hewitt (1880–1961), a Curator of the Sarawak Museum, Kuching between 1905–1908, refer to the species being described as new herein. Hewitt apparently did no field work locally (see Das and Leh 2005), and the museum ledger records collections (SM catalogue numbers D.a.2.4.2.a–k) made by his predecessor, Robert Shelford (1872–1912), who collected 11 specimens of Microhyla , allocated to M. achatina (now considered an endemic of the Malay Peninsula), seven of which were from the Matang Range, and some of these are suspected to be the source of Parker's (1934) material. More recently, a North American frog, Pseudacris crucife r has been suggested to be an associate of the North American pitcher plants, Sarracenia purpurea (see Russell 2008).
Discussion: The tadpoles of Microhyla nepenthicola sp. nov. exhibit an endotrophic mode of development, presumably exploiting the relatively stable (in terms of year-round availability of liquid contained therein) microhabitat provided by pitchers of Nepenthes ampullaria . They display the typical generalized microhylid ( Orton 1957: type II larvae) apomorphic features, such as non-perforated nostrils, terminal mouth, reduced oral disk, and midventral opening of the spiracle ( Haas 2003). However, endotrophy has presumably lead to noticeable morphological changes in this species, including relatively small size at metamorphosis, reduction of gill filter apparatus in terms of size and complexity (microhylids typically have a extensive branchial apparatus), rapid development, and gut functioning as yolk storage.
Miniaturization in amphibians is often accompanied by a suite of characters, including reduced ossification and reduction of digits ( Inger and Frogner 1980; Alberch and Gale 1985). Within the genus Microhyla , there is a tendency for reduction or loss of Finger I, and a few species show three functional fingers ( Inger and Frogner 1980). Miniaturization in this species and the reduced webbing on its pes may be the result of the need to navigate on the slippery (waxy) zone of pitchers of Nepenthes , situated below the peristome. The function of the waxy zone has been shown to be critical for trapping arthropods by these plants ( Gorb et al. 2005). Nepenthes ampullaria is somewhat unusual amongst other Nepenthes in having mostly lower pitchers, a diet comprising primarily of detritus and consequently, are found growing under canopy ( Clark and Lee 2004).
Other challenges in dramatic reduction in body size reported in poikilothermous tetrapods include increased rate of water loss, given their greater surface-to-volume ratios, compensated by their selection of the most humid microhabitats ( Hedges and Thomas 2001). The new Microhyla from Kubah is inactive during dry nights, and reproduction and other activities take place in the immediate vicinity of Nepenthes ampullaria , which is one of the smallest species within the genus, with pitchers up to 10 cm high and 7 cm wide ( Clarke 1997).
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.