Brachyteles hypoxanthus, Kuhl, 1820
|
publication ID |
https://doi.org/ 10.5281/zenodo.5727205 |
|
DOI |
https://doi.org/10.5281/zenodo.5727306 |
|
persistent identifier |
https://treatment.plazi.org/id/313A8814-2A0A-F32D-FA58-FCB968B9F6C6 |
|
treatment provided by |
Conny |
|
scientific name |
Brachyteles hypoxanthus |
| status |
|
25 View On .
Northern Muriqui
Brachyteles hypoxanthus View in CoL
French: Muriqui du Nord / German: Nordlicher Spinnenaffe / Spanish: Muriqui septentrional Other common names: Northern Woolly Spider Monkey
Taxonomy. Brachyteles hypoxanthus Kuhl, 1820 View in CoL ,
Brazil, Bahia.
Two subspecies were formerly recognized, arachnoides and hypoxanthus . C. P. Groves in 2001 classified them as separate species. Monotypic.
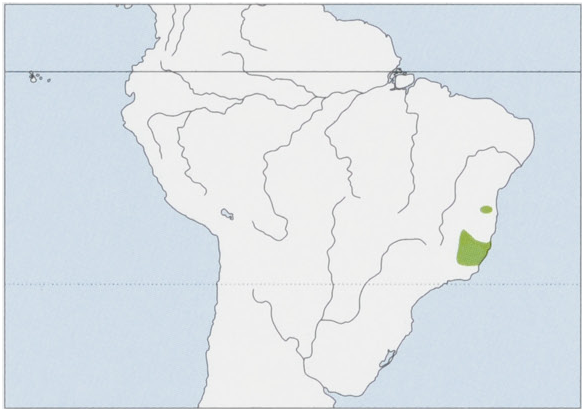
Distribution. Historically ranged through the Atlantic Forest in the states of Bahia, Espirito Santo, Minas Gerais, and Rio de Janeiro, excluding only lowland forests in the extreme S of Bahia and N Espirito Santo, the N limit of the distribution was probably the Rio Jequirica or the right bank of the Rio Paraguacu, and the S limit is still poorly defined but is probably the Serra da Mantiqueira in S Minas Gerais, near the state boundaries with Rio de Janeiro and Sao Paulo where it meets the distribution of the Southern Muriqui ( B. arachnoides ). View Figure
Descriptive notes. Head-body 47-8-49-7 cm (males) and 46-1-51-4 cm (females), tail 73-4-77-3 cm (males) and 73-8-81 cm (females); weight 9-2-9-6 kg (males) and 6-9-8-8 kg (females); sample of three individuals in all cases. These measurements were taken from living individuals captured in Fazenda Esmeralda, Rio Casca, Minas Gerais. Male and female Northern Muriquis are similar in size, and there is no sexual dimorphism in the size of their canines. Two male and four female specimens in the British Museum from Engenheiro Reeve, Espirito Santo, listed by P. H. Napier in 1976, were head-body 58-61 cm (males) and 54-5-60 cm (females), tail 67-69 cm (males) and 74-80 cm (females). Although muriquis are generally hailed as the largest New World monkeys, these measurements do not exceed those of large spider monkeys such as the White-bellied Spider Monkey ( Ateles belzebuth ) and the Red-faced Black Spider Monkey (A. paniscus ), or even a large male Central American Black Howler ( Alouatta pigra ). There is likely natural geographical variation in the body size of Northern Muriquis, and nutritional status and time of the year may result in disparate measurements. Pelage of the Northern Muriqui is very distinctive and predominantly beige, with light or dark brown or light gray-brown coloration. Northern Muriquis are born with a black face, but at sexual maturity, the face and genitalia lose their pigmentation and become spotty pink. Juveniles and adults have a pronounced swollen belly, accommodating extensive small and large intestines for the digestion of leaves. Adaptations for suspensory locomotion and tail-assisted brachiation include long arms, a metacarpal hook, and a long prehensile tail. Males have a large conspicuous scrotum, and females have an elongated cylindrical clitoris. Northern Muriquis have a vestigial thumb, which is diagnostic in differentiating them from Southern Muriquis that lack the thumb.
Habitat. Evergreen, dense, semi-deciduous and deciduous forest at 250-1350 m above sea level. The majority of the information available on the ecology, behavior, reproduction, and demography of the Northern Muriqui comes from the work of K. B. Strier and her colleagues since 1982 in seasonal, semi-deciduous forest in Caratinga Biological Station (Reserva Particular do Patrimonio Natural Feliciano Miguel Abdala), Minas Gerais. Habitats in Caratinga include relatively intact and mature forest (canopy 30-35 m), disturbed (degraded, logged) mature forest, swathes of secondary forest in advanced stages of succession (canopy 25 m), and young secondary growth. G. Fonseca in 1986 found that Northern Muriquis preferred late successional secondary forest. They used the intact forest as much as would be expected based on availability and avoided degraded mature forest. Contrary to expectations considering their size, they are not averse to entering young secondary growth—no doubt allied to their predilection for young leaves.
Food and Feeding. The diet of a group of Northern Muriquis monitored over 14 months at Caratinga was 51% leaves, 32% fruits (mostly ripe), 11% flowers, and bark, bamboo, buds, and ferns (6%). Diets varied with the availability and abundance of food resources throughout the year. Sixty three plant species from 57 genera were identified as food sources. They ate flowers of Apuleia praecox ( Fabaceae ) and Adenocalymma marginatum (Bignoniacae), and nectar and pollen of Mabea fistulifera ( Euphorbiaceae ). Diets were similar between the sexes, except that the females tended to eat more flowers.
Breeding. At Caratinga, breeding of Northern Muriquis is seasonal. Females usually disperse while still adolescent at c.6 years old (average 73-4 months, range 64-78 months). A female that stayed in her natal group had her first infant at ¢.7-5 years old, but age at first reproduction of dispersing females is prolonged. Entering another group takes a while, and following immigration, sexual activity is delayed a minimum of eleven months. Most females undergo puberty only after they have transferred to another group. On average, they give birth to their first infant at 33-34 months after joining the group. Ovarian cycles last c.21 days, but there are no visible signs of sexual receptivity. Pheromonal cues, especially from urine, are probably important, and males sniff the females’ genitalia. They pull on the females’ pendulous clitoris, which, rubbing on branches as it does, is probably important in distributing information about her sexual condition. Sexual interactions are concentrated in the early and middle wet season, and births peak in the dry season (April-September). Nearly two-thirds (66%) of 37 births recorded from 1982 to 1994 at Caratinga occurred in June-August and 86% in the dry season. Given c¢.7 months of gestation, conceptions are concentrated in the wet season (October—March). The interval between parturition and the first post-partum mating averages 23-5 months, and mating continues for an average of 5-6 months. Females rarely continue their sexual activity into the dry season, so those that do not conceive in the wet season can experience a year’s delay in reproduction (mating again only in the next wet season). Non-lactating females do not cycle throughout the year. Stimuli involved in the shut-down of ovarian cycling in the dry season may be shorter day lengths, declining female condition, or inclusion or exclusion of items in the diet that may affect fertility. Females that conceive early in the wet season (to give birth early in the dry season) resume sexual activity sooner (average 22-2 months) than females that conceive late in the wet season (23-9 months) or in the early dry season (24-3 months). Birth intervals average 36-4 months. Giving birth in the dry season is believed to be advantageous because it is not a period when fruit is abundant, which implies much traveling; relying on leaves, daily movements are shorter. Carrying an infant becomes more tiring as they grow, and mothers are able to benefit from increased food abundance in the early wet season when infants are getting larger but are still being carried most of the time. Male Northern Muriquis interact very little with infants. Weaning begins when infants are c.18 months old. Infants born in the dry season of one year are weaned in the wet season of the next year when fruit, young leaves, and flowers are abundant.
Activity patterns. Activity budgets of Northern Muriquis at Caratinga are relatively constant, averaging 49% resting, 29% traveling, 19% feeding, and 3% engaged in social and other activities. The pattern during the day is variable, but they there is a distinct habit of feeding more in the late afternoon. Resting and feeding are inversely related. They tend to be more active during the warmest hours of the day when it is cooler in the dry winter months (April-September), and they are more active in the coolest hours during the hotter summer months of the wet season (October-March). In the dry season, mature leaves make up a greater portion of their diet, and they travel less. In the wet season, when fruits, flowers, and young leaves are more abundant, they tend to travel more. Male and female activity budgets do not differ, but adults tend to rest more and immature animals are more active. Lactating females spend more time feeding than those without nursing infants. A 7-month study by R. Lemos de Sa of a group of 15 Northern Muriquis at the Fazenda Esmeralda recorded an activity budget of 62% resting (monthly range 56-68%), 18% feeding (11-24%), 16% traveling (11-24%), and 4% engaged in social and other activities (1-5%). Time spent feeding was similar to that found at Caratinga, but times spent resting (more) and traveling (less) were undoubtedly related to their smaller home range in a forest of only 44 ha. They spent more time feeding in the dry season (19-23% of the day) when food was scarcer than in the wet season when food was more abundant (9-15%).
Movements, Home range and Social organization. Northern Muriquis live in multimale—multifemale groups as a large as ¢.60 individuals. In a 14-month study at Caratinga (1983-1984), a group of 23-27 individuals, with six adult males and eight adult females, had a home range of more than 168 ha in an 800-ha forest. Their, daily movements averaged 1283 m (141-3403 m). About 46% of the home range was also used by a neighboring group. Home range use by this group was studied again in 1998-1999. It had grown to 57-63 individuals, including 13-14 adult males and 19 adult females. Home range size had increased to 309 ha. Daily movements remained quite constant despite the increase in home range size, averaging 1313 m (200-2835 m). At thissize, the group tended to split into subgroups that traveled and foraged independently. Subgroups were larger when food was more abundant in the wet season (average 41-8 individuals) than in the dry season (36-6 individuals) when fruits were scarce. Males generally stay in their natal group, but females disperse to other groups before they become sexually active. Intergroup encounters provide stimulus for emigrations and immigrations, and the process is evidently stressful. Females trying to enter a new group are not welcomed by resident females. They spend some time peripheral to the group and are chased and harassed. Sometimes they return for a time to their natal group. Even when tolerated, they tend to remain peripheral to a new group for some months, interacting most with the new group’s juveniles and adolescent males. Philopatric males have a high degree of relatedness among themselves, and certain individuals tend to consistently travel together. Females travel with their offspring of various ages. Northern Muriquis are promiscuous. Males and females are co-dominant, and they show no aggression in either sexual or non-sexual contexts. When aggression does occur, it is generally mild. Female choice probably makes it of little advantage for males to compete physically. Sexual competition is believed to be enacted through sperm competition rather than through overt contests. Males have large testicles that produce voluminous quantities of sperm.
Status and Conservation. CITES Appendix I. Classified as Critically Endangered on The IUCN Red List. Northern Muriquis are also classified as critically endangered on the Brazilian List of Threatened Species. Once widespread in the Atlantic Forest, their numbers are estimated at ¢.1200 individuals. Habitat fragmentation and hunting in the most heavily populated states of Brazil are the reasons for their decline. Threats to remaining populations include low genetic diversity and the isolation of the remaining populations. Today, they are found in fourteen separate populations, most of them formally protected. A national action plan was developed by the federal government in 2011, which sets out priorities and needs for research and conservation of Northern Muriquis. These include increasing habitat availability and providing connectivity among the forest patches where they survive. The Rio deJaneiro Primate Center has an effective breeding program, but with few individuals that were pets or rescues. There is no immediate reason to have individuals taken from the wild for captive breeding. A number of non-governmental organizations and the Brazilian government are working to save and nurture extant wild populations. Northern Muriquis occur in Caparao, Alto Cariri and Itatiaia national parks; Augusto Ruschi and Mata Escura biological reserves; Serra do Brigadeiro, Rio Doce, Ibitipoca, and Alto Cariri state parks; Mata dos Muriquis Wildlife Refuge; Reserva Particular do Patrimonio Natural Feliciano Miguel Abdala (Caratinga Biological Station), Mata do Sossego, and Fazenda Duas Barras private reserves. They are also found in a number of small, privately owned forest patches in Santa Maria de Jetiba, Espirito Santo. The extraordinary recovery of the population in Feliciano Miguel Abdala reserve, from c.40 individuals in the early 1980s to well over 200 individuals today, testifies to the resilience of the Northern Muriqui, given adequate protection and space.
Bibliography. Aguirre (1971), Boubli et al. (2010), Carvalho et al. (2004), Coimbra-Filho et al. (1993a), Ferrari & Strier (1992), da Fonseca (1986), Groves (2001), Hill (1962), Lemos de Sa (1988), Lemos de Sa & Glander (1993), Lemos de S4, Pope, Glander et al. (1990), Lemos de S4, Pope, Struhsaker & Glander (1993)), Jerusalinsky et al. (2011), Martins & Strier (2004), de Melo & Dias (2005), de Melo et al. (2004), Mendes et al. (2005), Mittermeier et al. (1987), Napier (1976), Nishimura et al. (1988), Pissinatti (2005), Ruschi (1964), Strier (1987a, 1987b, 1991, 1996, 1997, 1999b, 2000), Strier & Boubli (2006), Strier & da Fonseca (1997), Strier & Ziegler (1997, 2000), Strier, Boubli et al. (2006), Strier, Dib & Figueira (2002), Strier, Mendes & Santos (2001).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.