Lispe Latreille, 1797
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4557.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:330BE81C-E3E0-4CA5-9017-DFB203EB7329 |
|
DOI |
https://doi.org/10.5281/zenodo.5934163 |
|
persistent identifier |
https://treatment.plazi.org/id/3219654C-FFEF-FFDC-37E8-51342468FAEB |
|
treatment provided by |
Plazi |
|
scientific name |
Lispe Latreille, 1797 |
| status |
|
Genus Lispe Latreille, 1797
Lispe Latreille, 1797: 169 . [Without included species.] Type-species: Musca tentaculata De Geer, 1776 , by subsequent
monotypy ( Latreille, 1802: 462). Gender: feminine. Lispa Walckenaer, 1802: 392 . Emendation of Lispe Latreille, 1797 . Xenolispa Malloch, 1922a: 279 (as a subgenus of Lispa [sic] Latreille, 1797). Type-species: Lispa (Xenolispa) atrifrontata
Malloch, 1922 [= Lispe sydneyensis Schiner, 1868 ], by original designation. Gender: feminine. Chaetolispa Malloch, 1922b: 386 . Type-species: Lispa geniseta Stein, 1909 , by original designation. Gender: feminine.
Diagnosis: Species of the genus Lispe can immediately be recognised by the dilated palpus (e.g. Fig. 19 View FIGURES 16–20 ) and setulose anepimeron. Almost all species have a setulose abdominal sternite 1, a partly to wholly setulose parafacial, and a tuft of short black setulae on the hind margin of posterior thoracic spiracle.
Description: Frons broad in both sexes, usually strongly convex and narrowing to just below lunula (e.g. Fig. 18 View FIGURES 16–20 ). Eye bare; with an area of facets alongside lunula enlarged, more marked in ♂ than in ♀. Ocellar setae strong (fine and short in L. nigrimana , L. nigrimanoides , L. flaveola , L. sydneyensis , L. nicobarensis , and a few others, especially ♂♂). Inner verticals long, generally twice as long as outer verticals. Fronto-orbital plate narrow. 2 pairs of reclinate orbital setae, lower pair absent in L. nigrimana , L. flaveola and sometimes in a few others; 3 or more pairs of inclinate frontal setae. Fronto-orbital plate with short setulae that continue down parafacial almost to lower eye-margin, parafacial rarely bare in upper part or, very occasionally, even entirely bare. Frontal triangle reaching lunula. Palpus swollen in apical part, spoon- or saucer-shaped. Proboscis dark brown, prementum glossy. Acr setae absent, rarely with a weak prsc pair. Pra absent. 2 pal, anterior one at most half as long as posterior one. Prosternum bare. Proepisternal depression bare. Notopleuron with 2 setae, second one the shortest, otherwise bare. Anepimeron with fine setulae on lower part. Subalar bulla bare. Posterior spiracle small, round, with at least 2 strong black setae on posterior margin, but these sometimes inconspicuous, and apparently absent in glauca . Katatergite with short pile. Anatergite bare. Scutellum with strong apical and sub-basal lateral pairs of setae. Mid femur without ad preapical seta (except in L. setigena and L. pygmoza ). Hind tibia with calcar absent; usually with strong d and ad preapical setae, but either one of these sometimes absent. Wing-membrane entirely covered with microtrichiae. Veins bare, except costa; costa setulose ventrally almost to tip of vein R 2+3. Vein A 1 +CuA 2 extending for over half the distance from crossvein CuA 2 to wing-margin. Lower calypter of the " Phaonia - type ". Sternite 1 with fine setulae at least at sides (very short or absent only in L. leucospila ).
Characters of the male and female terminalia are discussed below (under L. pygmaea , L. tentaculata and also under each species). As a general comment, in most males the epandrium is produced ventrad into a “pseudosurstylus”, although without any articulation. Sternites 1–3 or 1–4 do not usually differ between the sexes, whilst in dry specimens sternite 5 is at least partly concealed beneath sternite 4. The female ovipositor is rarely much longer than the length of segment 5, and the three spermathecae are subspherical.
Taxonomic position. For many years Lispe was usually placed in its own subfamily (e.g. Malloch, 1934; Emden, 1941, 1965; Huckett, 1965), which emphasised its autapomorphies without illuminating its relationships. Karl (1928) had placed Lispe as the sister-group of the rest of his Limnophorini , but it was Hennig (1960a, 1965) who first justified this assignment in a phylogenetic sense by pointing out that the ground-plan characters of Lispe are those of the Limnophorinae, i.e. lower calypter tongue-shaped, of the Phaonia - type, hind tibia without a calcar, prealar seta absent. The genus is now usually placed in the tribe Limnophorini of the subfamily Coenosiinae ( Pont, 1986, 1989; Carvalho et al., 2005) or in a tribe Lispini alongside the tribes Limnophorini and Coenosiini of the subfamily Coenosiinae ( Fan, 2008; Xue & Chao, 1998).
Adaptations. Adults of Lispe generally fly low and rapidly close to the ground, and run rapidly and jerkily when on the ground. They mate on the ground, and male courtship displays are terrestrial rather than aerial and are based on visual and tactile signals. As aggressive predators they also spend much time on the ground, stalking prey on foot and then pouncing by means of a short flight. Several morphological adaptations reflect these largely cursorial habits, for example: a relatively short wing to body ratio, broad frons in both sexes, enlarged upper inner eye facets, and robust legs with strong tarsi and well developed apical tibial setae.
Species-groups. Snyder (1954) and Hennig (1960a) were the first to propose species groups within Lispe , but it is only recently that additional species groups have been proposed and defined ( Vikhrev, 2011a, 2011b, 2012b, 2014, 2015, 2016; Vikhrev, Ge & Zhang, 2016). These have been based mostly on the chaetotaxy of the body and legs, sometimes combined with external features of the male terminalia. Little or no use has yet been made of the internal male genitalia (phallic complex) or of the female ovipositor, nor of the very remarkable postabdominal segmentation. For the most part, the groups are based on practical convenience, as a means of breaking up this speciose genus into smaller, manageable units. Few of them are defined in monophyletic terms as virtually no attempt has been made to determine what are plesiomorphous character states, what are apomorphous character states, and what characters are synapomorphies. In the case of the leg chaetotaxy this is impossible to determine, but some apomorphies (in terms of the ground-plan of the genus) can be identified. Until a broad-based cladistic analysis of the whole genus has been made, it will not be possible to determine which groups are monophyletic or to determine evolutionary trends within the genus.
Phylogeny. I suggest that a species like Lispe pygmaea Fallén, 1825 is closest to the ground-plan of the genus, and this can be supported by the weak expansion of the palpus, the full development of the scutal and katepisternal setae, and, in the male terminalia, the retention of freely articulated surstylus and a relatively simple aedeagal complex ( Figs 2–5 View FIGURES 2–5 ); in the female terminalia, there are few if any spines on the hypoproct ( Figs 6–8 View FIGURES 6–8 ).
Characters within Lispe which appear to be apomorphous are as follows:
(1) 1 or 2 strong setae on the lower parafacial.
(2) Reduction in the number and strength of the dorsocentral setae.
(3) Reduction in the number and strength of the katepisternal setae.
(4) Presence of a group of setulae on meron, below the spiracle.
(5) Presence of a strong setula on the posterior surface of hind coxa.
(6) Progressive reduction of the male postabdominal segments.
(7) Reduction and loss of the male surstylus.
(8) Ventral expansion of the male epandrium to form a surstylus-like extension.
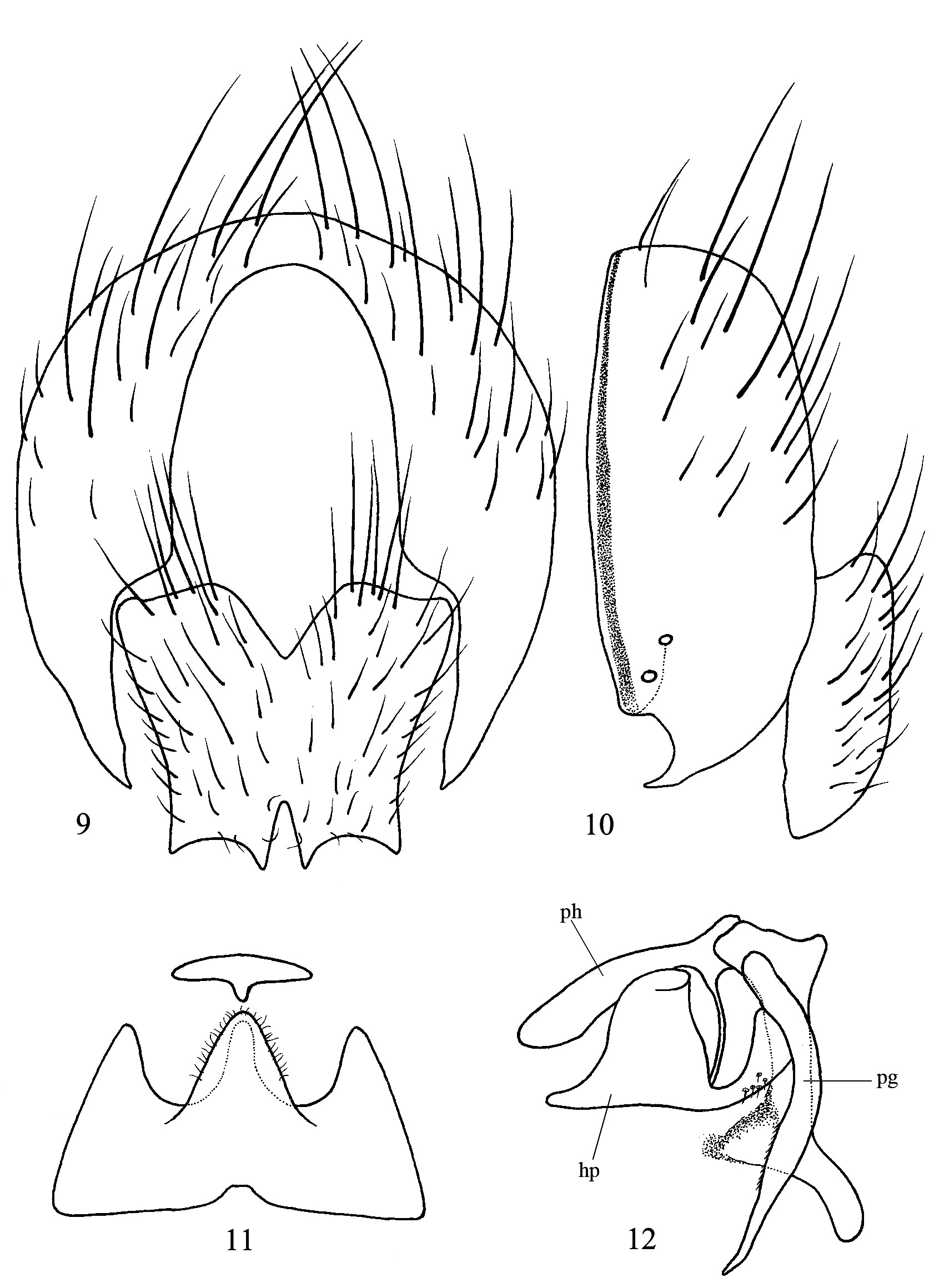
The most interesting of these is undoubtedly the reduction of the postabdominal segments, which reaches its most extreme form in the common Palaearctic Lispe tentaculata (De Geer, 1776) . Here the postabdominal segments have been completely lost, tergite 5 is followed by the epandrium, and the postabdominal spiracles are situated in the epandrium ( Figs 9–10 View FIGURES 9–12 ). Curiously, this feature has never been remarked before. In many other species, including those dealt with in this paper, these postabdominal segments are reduced to varying degrees, often strap-like and bearing the postabdominal spiracles.
The following is a brief description of the male and female terminalia of Lispe pygmaea Fallén, 1825 , which will illustrate the more plesiomorphous morphological features of the genus:
♂. Epandrium and tergite 5 separated by syntergosternite 8 which is large, symmetrical, band-like, with 2 spiracles, bare, with no evident lines of fusion or division. Sternite 6 present as a small sclerite after/below sternite 5. Surstylus distinct, free-lying; upper anterior angle attaches to hypandrium, upper posterior angle attaches to upper inner corner of cercal plate. Cercal plate small, deeply divided. Hypandrium attaching at lower anterior corner of epandrium and to surstylus; bearing two bars and a shield-like plate that overlies phallus. Phallic complex: praegonite absent; epiphallus absent; phallapodeme shaped like an inverted V (bifid), connected by a small rod to a narrow plate which in turn connects with postgonite; postgonite long, strong, with a membranous posterior edge; phallus strongly sclerotised, with abundant and powerful spines and spinules on juxta, near base with an anterior projection and also with a posterior rod that lies against the plate lying between phallapodemal rods and postgonite.
♀. Tergite 6 complete dorsally; tergite 7 divided dorsally; tergite 8 divided dorsally, with setulae and a spinule at lower angle. Sternites 6 and 7 complete; sternite 8 present as 2 narrow plates. Epiproct complete, large; with a pair of long setae. Hypoproct large, apical part produced and turned upwards, with a few weak spines. Cercus setulose, with a single spine. 3 spermathecae.
This can be compared with the following description of Lispe tentaculata (De Geer, 1776) , type-species of the genus Lispe .
♂. Epandrium attached to tergite 5, the intermediate tergite 6 and syntergosternite 8 lost; with 2 spiracles at anterior lower edge; apparently a fusion product. Sternite 6 present as a small sclerite after/below sternite 5. Surstylus fused with epandrium on outer epandrial surface, with no line of fusion or division visible on the outside; on inner surface of epandrium, upper part of "surstylus" growing free from epandrial wall and its upper margin attacheinh anteriorly to hypandrium and posteriorly to cercal plate. Cercal plate large, quadrate, with a short division before apex. Hypandrium with only a single attachment, to anterior end of surstylus; with a median prolongation that reaches to and joins base of phallapodeme. Phallic complex relatively simple: praegonite absent, though its former presence indicated by sensory pores at posterior edge of hypandrium; epiphallus absent; phallapodeme moderate, narrow; postgonite long, strong, sickle-like; phallus a simple tube, without apparent juxta or spinulation.
♀. Spiracles 1–5 becoming progressively larger, diameter of spiracle 5 at least twice that of spiracle 1. Tergites 6 and 7 divided dorsally; tergite 8 complete dorsally, expanded below. Sternite 6 divided, present as 2 small plates; sternite 7 a single plate; sternite 8 a single large plate. Epiproct divided into 2 small plates, with only setulae. Hypoproct large, apical part produced and turned upwards, with several spines. Cercus setulose, with a single spine. 3 spermathecae.
Biology. All species of the genus Lispe are carnivorous in the larval and adult stages. Indeed, this feature is a ground-plan character of the entire subfamily Coenosiinae ( Limnophorini + Coenosiini ) ( Couri & Pont, 2000: 374).
What is known of the biology has been summarised by Skidmore (1985), who has also given descriptions of the immature stages of the few species for which these are known. The eggs have the hatching pleats produced anteriorly into respiratory horns which are ornately papillate. Larvae hatch in the second instar and develop in wet sand or mud with a high organic content, in algal mats in ponds and ditches, and also in shore debris. L. orientalis Wiedemann has been reared in Pakistan from cow dung, according to label data on specimens in the BMNH. Larvae feed on other subaquatic dipterous larvae ( Tipulidae , Chironomidae , Ceratopogonidae , Dolichopodidae , Ephydridae ), Phyllopoda, etc. Pupariation takes place in the substrate or in the immediate vicinity.
Adults have been reported as taking a variety of small Diptera as prey such as Chironomidae ( Charbonnier, 1918; Wirth, 1947), Psychodidae and Milichiidae ( Williams, 1938) , and Tephritidae and Ephydridae ( Hardy, 1981) . They have attracted attention in tropical areas of the Old World as predators of Culicidae and Simuliidae , preying on larvae, puparia as well as adults. Lispe predation on culicid larvae and adults has been described in Hong Kong ( Atkinson, 1909), in Hawaii ( Williams, 1944), and in Africa by Lamborn (1920) and Cuthbertson (1937). Lispe nivalis Wiedemann was noted as a major predator of young adult Simulium damnosum Theobald and Simulium adersi Pomeroy in Haute-Volta ( Balay & Grenier, 1965) . In Europe, adult Lispe loewi Ringdahl prey on Aedes dorsalis (Meigen) (Culicidae) and Culicoides longicollis Glukhova (Ceratopogonidae) ( Szadziewski, 1982). The mating and predatory behaviours have been described in detail for Lispe tentaculata (De Geer) by Schlee (1977) and for Lispe candicans Kowarz by Steidle et al. (1995). A summary of the predaceous activity was given by Werner & Pont (2006). Further more recent information and photographs of the adults with prey are given in the papers of Vikhrev (2011a, 2011b, 2012b, 2012c, 2014, 2015, 2016) and Werner, Kampen & Pont (2015).
Observations in Australia are sparse in the extreme. No life-histories are known, despite the abundance of species (and individuals) especially in littoral habitats. The type-series of L. nigrimana was reared from puparia found in mud at the edge of the Burnett River. L. geniseta , the sister-species of L. setigena , was reared in Indonesia from rice stems ( Stein, 1920: 61). In his study of Australian kelp flies, McAlpine (1991: 82) wrote that predators of coelopid larvae probably included, among others, muscid larvae such as Lispe , and also reported that he had observed adults of a Lispe species eating adults of This canus McAlpine ( Coelopidae ) and other small, beach-living flies. Pont (1973a: 197) and Pont & Werner (2006: 98) reported on the muscid Neomyia lauta (Wiedemann) taken as prey by Lispe setigena (as geniseta , misidentification).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Lispe Latreille, 1797
| Pont, Adrian C. 2019 |
Lispe
| Latreille, P. A. 1797: 169 |