Phymonotus jancintotopos Lightfoot, Weissman and Ueshima
|
publication ID |
https://doi.org/ 10.5281/zenodo.203746 |
|
DOI |
https://doi.org/10.5281/zenodo.5622551 |
|
persistent identifier |
https://treatment.plazi.org/id/321F87B8-C940-FF80-FF5D-F983B4CEF7C9 |
|
treatment provided by |
Plazi |
|
scientific name |
Phymonotus jancintotopos Lightfoot, Weissman and Ueshima |
| status |
sp. nov. |
Phymonotus jancintotopos Lightfoot, Weissman and Ueshima new species
Figures 1 View FIGURE 1 , 2 View FIGURE 2. a , 3 View FIGURE 3 , 4 View FIGURE 4 , 5 View FIGURE 5. a , 6 View FIGURE 6. a , 7 View FIGURE 7 , 8 View FIGURE 8 ; Tables 1 View TABLE 1 , 2 View TABLE 2 .
Holotype. Male. ( Figs 1 View FIGURE 1 a, b) USA, California, Riverside County, San Jacinito Mountains, Fern Valley, Idyllwild, intersection of Fern Valley Road and Dickinson Road N 33° 45’ 22.39” W 116° 41’ 59.21”, 1,767 meters elevation, 31 August 1986, coll. D.B. Weissman, B.I. Weissman, D.C.F. Rentz, DBW stop number S86-105, song recording number R86-202, testes preserved for chromosome analysis sample number T86-86. Type deposited in CAS, number18565.
Paratypes: USA: California: Riverside Co: Fuller Mill Creek nr. Idyllwild, 6 September 1969, coll. P. Rauch, S. Larish, 13 (in ethanol), CAS; trail above Idyllwild, elev. 5,500 ft., 25 December 1969, coll. J. Emmel, O. Shields, 33, CAS; Idyllwild; elev. 1,950 m, 12 December 1982, coll. K.W. Cooper, 1Ƥ, CAS; San Jacinto Mountains, Idyllwild, 27 August 1983, coll. S. Bennett, DBW stop number S83-120, 1Ƥ, immature, ( CAS); San Jacinto Mountains, Cinco Poses Springs, elev. 7,200 ft, 4 September 1985, on ponderosa pine trees, coll. D. Goodward, 23, 2Ƥ, CAS; San Jacinto Mountains, intersection of Fern Valley & Dickinson Roads, elev. 5,900 ft, 31 August 1986, coll. D.B. Weissman, B.I. Weissman, D.C.F. Rentz, DBW stop number S86-105, song recordings R86-105, R86-192, R86- 196, R86-202, R86-203, R86-206, R86-219, R86-120, testes preserved for chromosome analysis T86-81, T86-82, T86-86, T86-87, T86-90, T86-91, T86- 92, 12 3 (including holotype), 12Ƥ, CAS; Boulder Basin Campground, 15 mi NW of Idyllwild, 33.826º N, 116.755º W, elev. 2,498 m, 6 October 2001, coll. J.A. Cole, 73, 1Ƥ, MSB; San Jacinto Mountains, Idyllwild, intersection of Fern Valley & Dickinson Roads, N 33° 45’ 22.39” W 116° 41’ 59.21”, elev. 1,767 m, 31 August 2005, on Jeffery pine trees, coll. D.B. Weissman, D.C. Lightfoot, DBW stop number S05- 114, 13, 1Ƥ, MSB.
Diagnosis. Monotypic. See Table 1 View TABLE 1 and diagnosis for the genus Phymonotus to distinguish P. jacintotopos gen et sp. nov. from nearest relatives.
can Nedubini . Phymonotus is unique for characters marked with an asterisk.
Character Gender Neduba Agalothorax Phymonotus
* Character states unique to Phymonotus .
Etymology. The genus name Phymonotus is the Greek prefix “ phymo ” for swollen, and the Greek suffix “ notum ” in recognition of the unusually enlarged, dome-shaped pronotum. The species name jacintotopos is composed of “ Jacinto ” for the Spanish name of the San Jacinto Mountains, and the Greek suffix “ topos ” for place, recognizing the San Jacinto Mountains, where the genus and species are endemic. Pronounced in English “haw-sintoe-toe-pose.” We recommend the common name: “San Jacinto shield-backed katydid.”
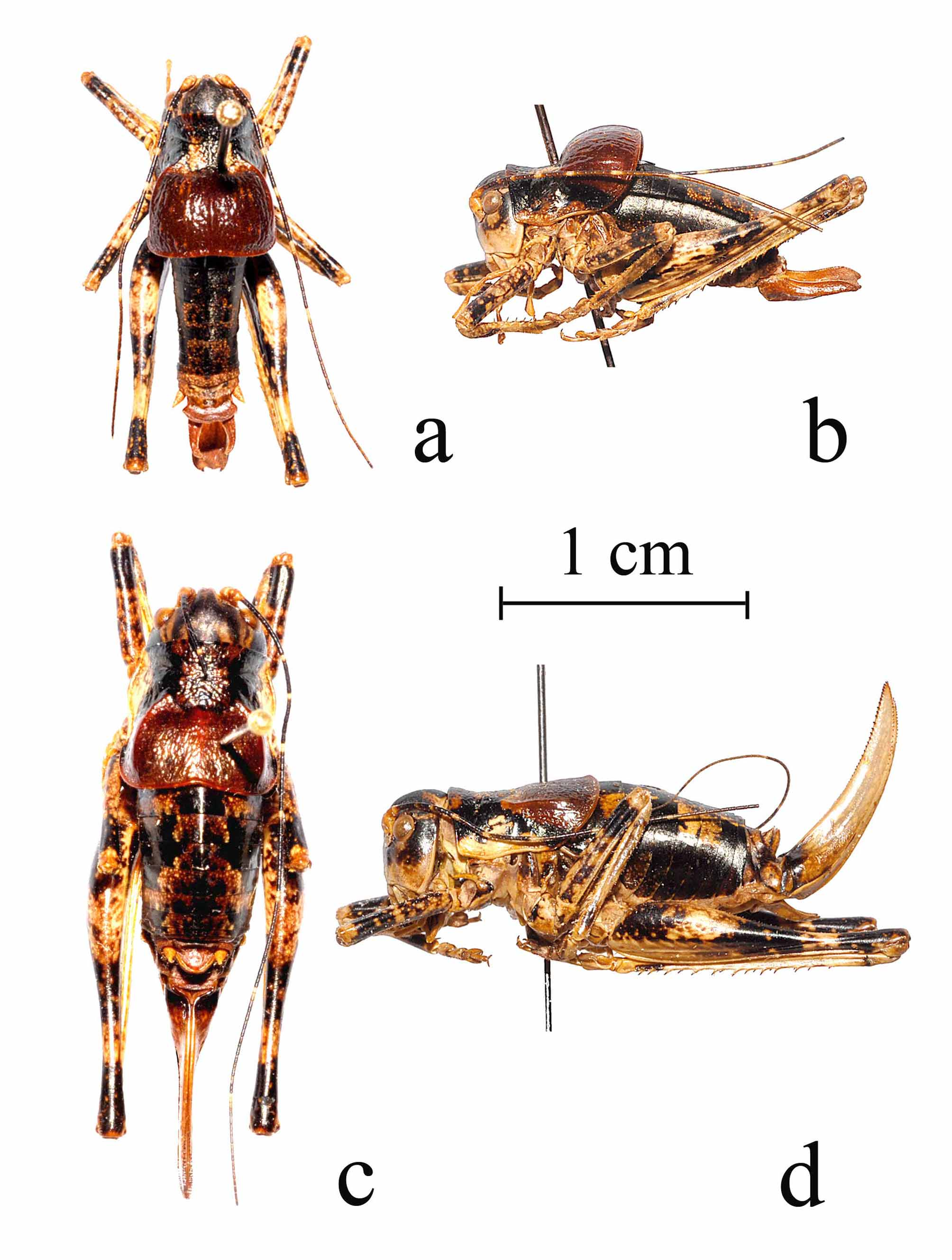
Description. General ( Figs 1 View FIGURE 1 , 2 View FIGURE 2. a ). Body robust, size medium compared to other North American Tettigoniinae , similar in size and build to other Nedubini , brachypterous. Body lengths of males 13.7–18.2 (15.5) mm (holotype male measurements in parentheses, see Table 2 View TABLE 2 for average measurements) and body lengths of females 13.5–20.9 mm. Females are generally larger than males, except for the dimensions of the metazona, which are larger in males.
Head ( Fig 2 View FIGURE 2. a ). Frons flat, weakly oblique, smooth and shiny, mid-dorsally acute and raised anteriorly to a rounded point between the eyes, equal in height to the height of the sclerotized base of the antennal socket above the circumantennal sulcus; gena smooth, shiny, and broadly rounded; basal width of dorsal head margin 3.9–5.5 (4.8) mm in males, and 4.0– 5.7 mm in females. Dorsal head length from posterior margin to anterior tip of vertex 1.7–2.8 (2.1) mm in males, and 1.8–3.3 mm in females; anterior apex of vertex elevated to the height of the antennal scape folded back against the head, gradually lowering to the occiput and posterior margin of the head; width of vertex between the eyes 0.5–0.7 (0.5) mm in males and 0.5–0.8 mm in females, fastigium oval and concave anteriorly, forming a shallow depression, rounded anteriorly, and acute posteriorly, equal in width to the antennal pedicel; slightly broader and shallower in females than males; top of head with dull, smooth surface, distance between the eyes 2.2–2.8 (2.6) mm in males and 2.2–3.2 mm in females; eye round, slightly extended subanteriorly when viewed laterally, ventral/dorsal height 1.1–1.3 (1.3) mm in males and 1.2–1.6 mm in females, and anterior to posterior width 1.0–1.2 (1.2) mm in males and 1.0– 1.4 mm in females; ocelli absent. Antennae filiform, about 1.5 times the body length ( Fig 1 View FIGURE 1 ).
1Teeth along dorsal margin of dorsal valve; 2Teeth along ventral margin of dorsal valve; 3Teeth along ventral margin of ventral valve; 4The measurement does not apply, wrong gender.
Thorax ( Fig 2 View FIGURE 2. a ). Prozona of pronotum relatively flat on dorsal surface, upcurved along anterior dorsal margin, and strongly upcurved posteriorly toward the metazona, dorsal anterior margin of prozona slightly concave at median, entire margins of prozona represented by a carina throughout, ventral margin of prozona flared outward laterally, and forming a prozonal lateral lobe, distinct from the metzonal lateral lobe, dorsal length of prozona 2.1– 2.9 (2.8) mm in males and 2.3–3.6 mm in females, dorsal width of prozona 1.6–2.5 (2.1) mm in males, and 1.8–2.5 mm in females, ventral width of prozona 4.8–6.5 (5.9) mm in males, 4.9–7.1 mm in females, dorsal to ventral depth of prozona 2.1–3.1 (2.7) mm in males, 2.5–3.6 mm in females, dorsal lateral carina absent on most of prozona, but abruptly appearing posteriorly, and extending to the metazona, principal anterior lateral sulcus of prozona narrow and shallow dorsally, becoming very deep and broad laterally to the ventral margin, secondary posterior lateral sulcus of prozona absent dorsally, shallow below lateral carina, extending ventrally forward and converging with principal sulcus above ventral margin of prozona, forming a slightly concave lateral face of the prozona mid-way to the ventral margin, above the lateral lobe, lateral margin of dorsal surface of prozona with a single to several flat tubercles present on posterior margin of principal lateral sulcus, surface of prozona rugose and shiny; metazona greatly inflated and produced dorsally as a rounded bulbous dome, encompassing both the dorsal disk and the lateral lobe, especially in males, averaging 1.5 times the height of the prozona, less so in females, averaging nearly equal to the height of the prozona; lateral lobe of metazona distinct from the prozonal lateral lobe; posterior margin of metazona strongly and broadly concave, especially in males, metazona dorsal length 2.7–5.5 (4.5) mm in males, 2.7–4.0 mm in females, metazona dorsal width 4.4–6.8 (5.9) mm in males and 4.6–6.6 mm in females, metazona ventral width 5.6–7.1 (6.3) mm in males and 5.3–7.4 mm in females, dorsal to ventral depth of metazona 3.2–5.0 (4.0) mm in males and 2.2–3.7 mm in females, dorsal median carina of metazona pronounced and entire, but only slightly elevated, lateral carina of metazona pronounced, entire, and slightly elevated, terminating just before the intersection with the posterior margin, outer margins of metazona with numerous slightly elevated carina throughout, converging with the same carina of the prozona margin, surface of metazona shiny with numerous anterior/posterior oriented low rugose ridges on dorsal disc and lateral margins; prosternum narrow and U-shaped with one broad spine on each side inside of the front coxae; mesosternum and metasternum both rectangular, with upturned lateral margins just inside of the middle and hind coxae respectively.
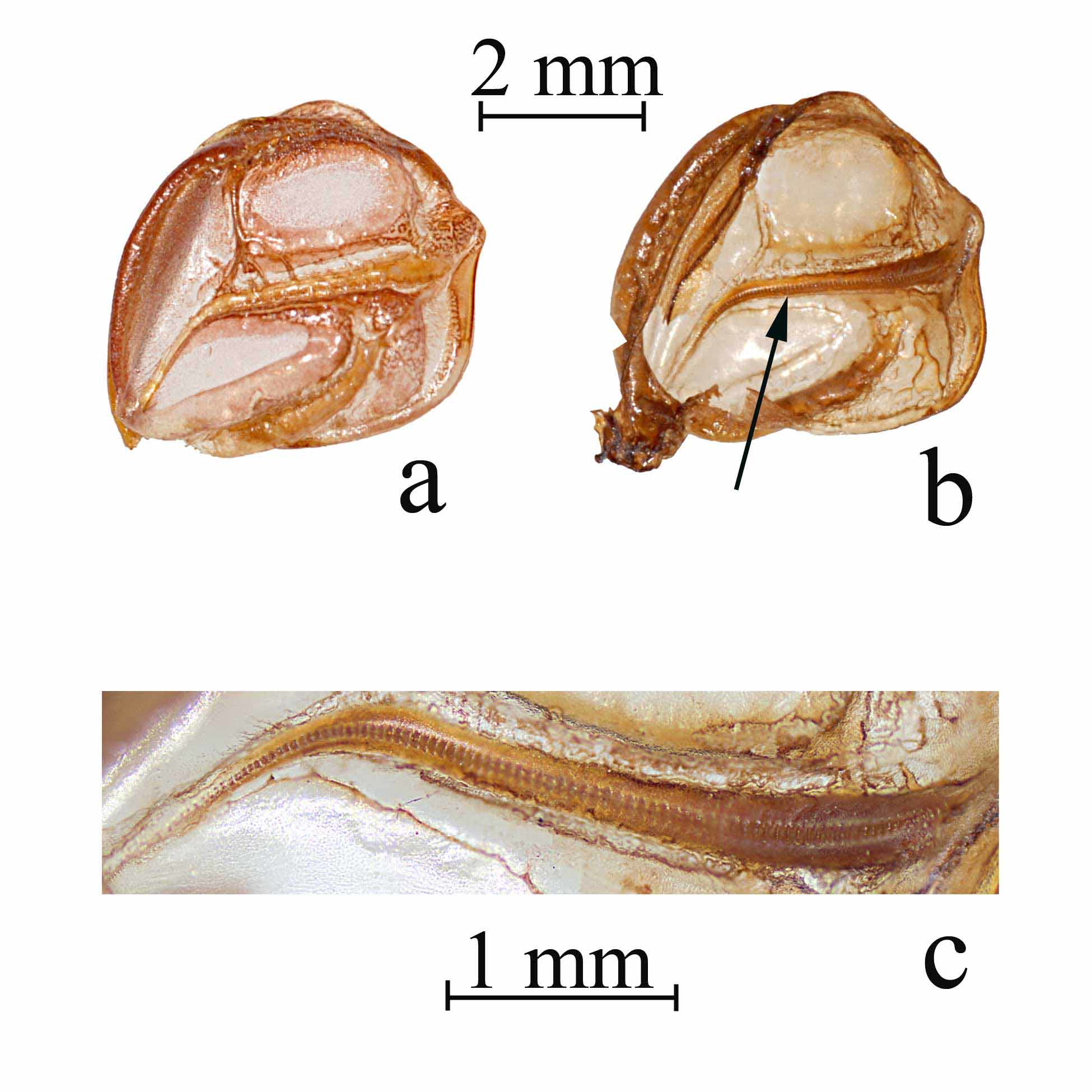
Wings ( Fig 4 View FIGURE 4 ). Brachypterous, wings hidden under the pronotal disk ( Figs 4 View FIGURE 4 a, b); stridulatory file of male wide and straight in the anterior half, curving laterally and tapering to a point in the posterior half ( Figs 4 View FIGURE 4 b, c), 3.1–3.9 (3.4) mm long; all teeth similar in thickness, but becoming narrow in the posterior portion; tooth count ranging from 81–111 (85).
Legs ( Fig 2 View FIGURE 2. a ). Front coxa with a large broad tooth on anterior base pointing downward, middle and hind coxa simple with no armature or extensions. Tympanum on front tibia beneath two narrow longitudinal lateral openings on external and internal margins; front tibia with 6 short spines on both ventral margins, one spine on outer margin at apex, and one spine on middle dorsal margin above the tympanum; front femur with one very small spine on inner ventral margin at apex; middle tibia with 7 short spines on both ventral margins, 3 short spines on both dorsal margins; hind tibia with 13 short spines on both dorsal margins, 5 short spines on both inner and outer ventral margins, and one large apical spin on both inner and outer lateral margins; hind femur length of males 10.0–14.5 (13.9) mm in length, and 2.1–3.2 (2.9) mm in width, hind femur of females 10.7–16.0 mm in length and 2.1–3.2 mm in width, with series of small teeth along the dorsal surface of the dorsal valve near the apex, a series of small teeth on the ventral surface of the ventral valve near the apex especially near the middle 1/3.
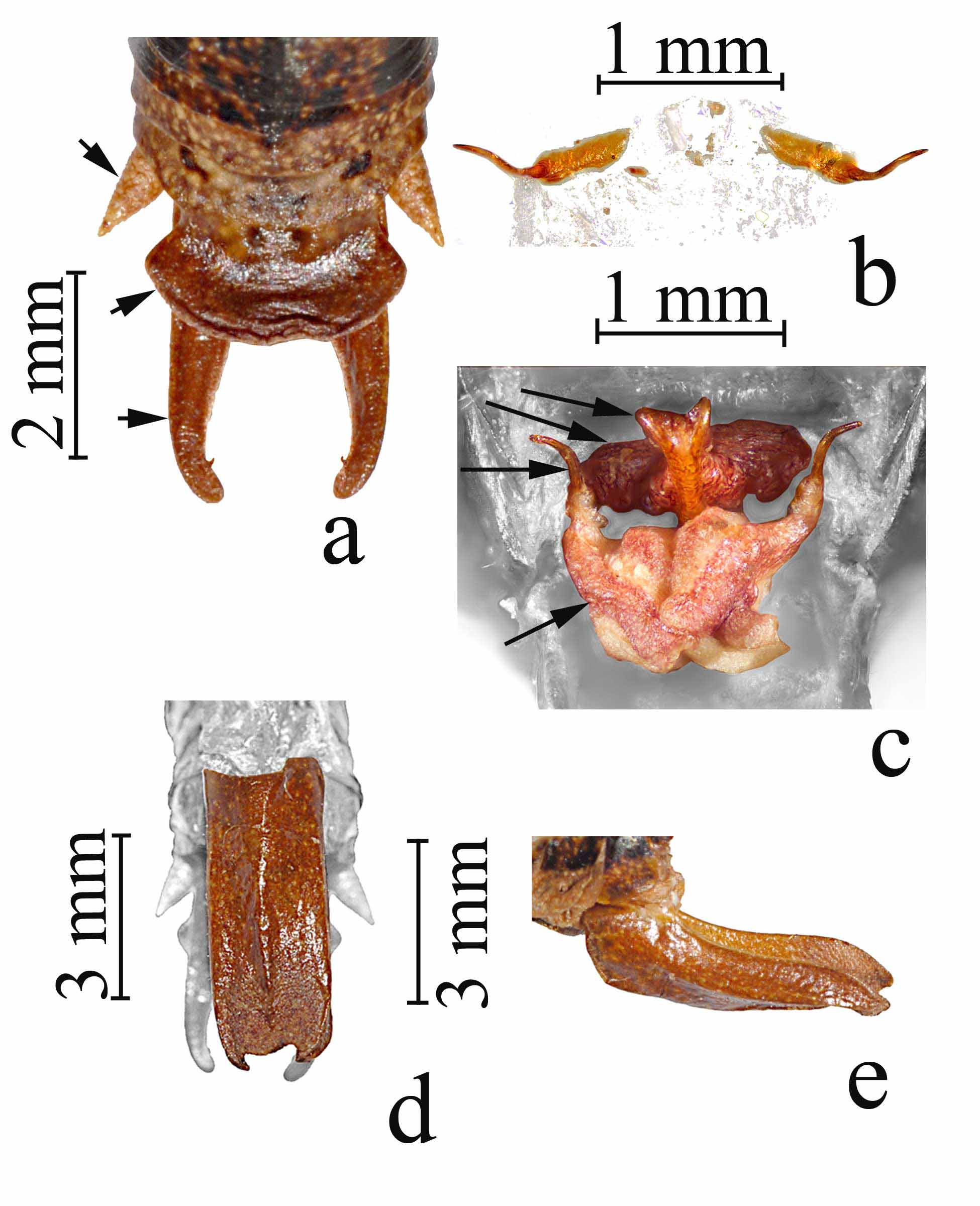
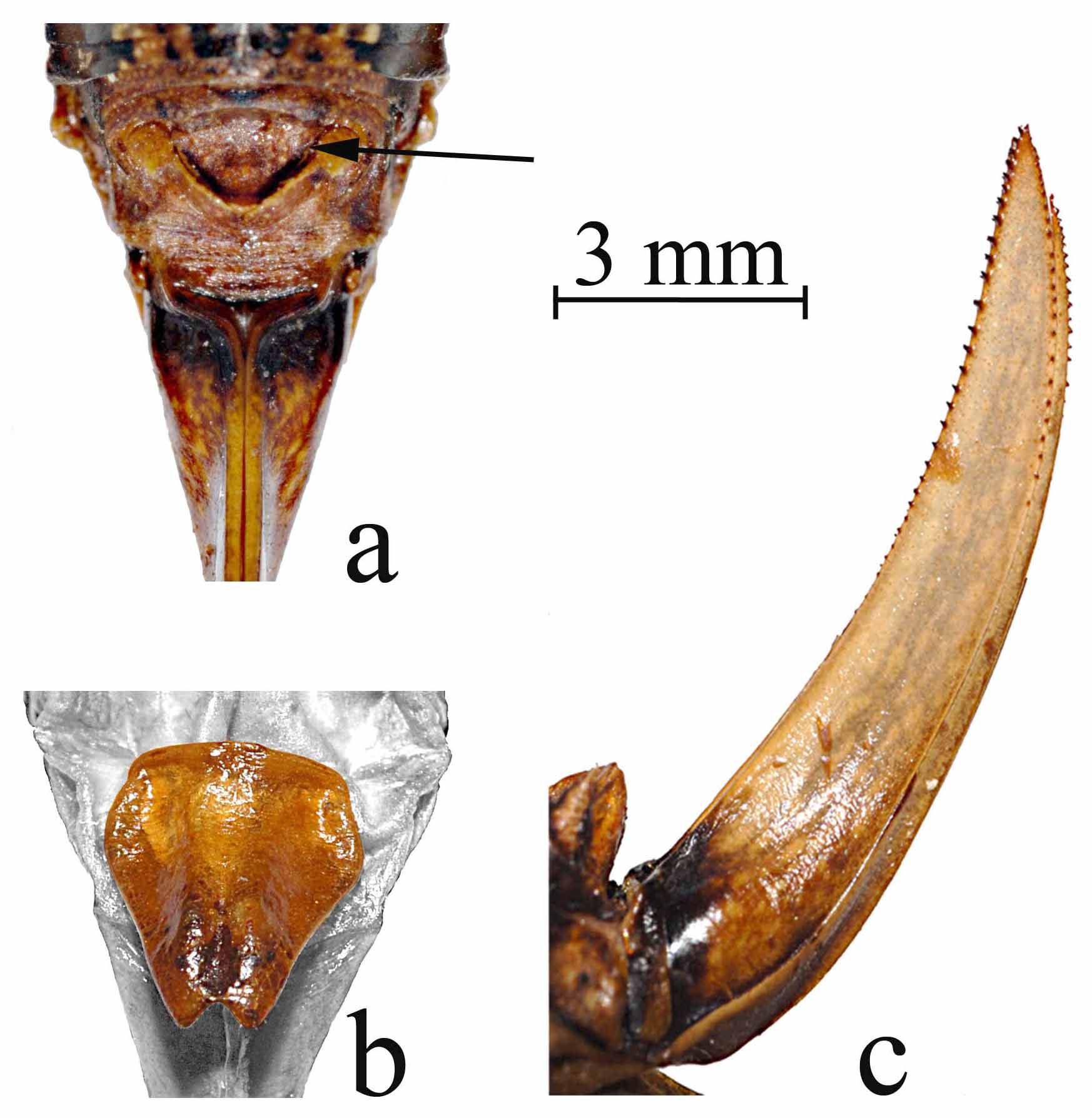
Abdomen ( Figs 2 View FIGURE 2. a , 5 View FIGURE 5. a , 6 View FIGURE 6. a ). Cercus short, simple styliform ( Fig 5a View FIGURE 5. a ); supra-anal plate (epiproct) of male large, broad basally, constricted medially, and then broad again distally, forming an hour-glass shape, and dorsally covering the basal 2/3rds of the paraprocts beneath ( Fig 5a View FIGURE 5. a ); supra-anal plate of female broadly triangular, broadest basally ( Fig 6a View FIGURE 6. a ); paraprocts of male long, narrow and dorsal/ventrally flattened, broad basally, narrowing to a rounded apical point that is strongly incurved with a small stout tooth located interior just below the apex ( Fig 5a View FIGURE 5. a ); titillator of male simple, arm sclerotized, slender and cylindrical, tapering to dorsal-laterally divergent apical point relative to the other titillator arm, sclerotized titillator arm separated from the right titillator arm by a non-sclerotized membranous base that is subequal to the length of a single arm ( Fig 5 View FIGURE 5. a b); we here name and describe the dorsal and ventral lobes of the titillators, and the dorsal sclerites of the titillators, all located between the supra-anal and subgenital plates ( Fig 5 View FIGURE 5. a c, colored features, gray background areas are the supra-anal and subgenital plates), the dorsal lobe being fleshy basally ( Fig 5 View FIGURE 5. a c, second arrow from top) (note that the dorsal lobe illustrated is dry and withered, it is full and turgid in live specimens) and supporting a sclerotized lobed structure that we name the dorsal sclerite of the titillator ( Fig 5 View FIGURE 5. a c, top arrow), projecting dorsally and anteriorly above the titillator ( Fig 5 View FIGURE 5. a c, third arrow from top shows apical tip of the left titillator arm); a ventral sclerite of the titillator as in Neduba is lacking, instead represented only by a fleshy lobe ( Fig 5 View FIGURE 5. a c, bottom arrow) that we here name the ventral lobe of the titillator (note that the ventral lobe illustrated also is dry and withered, it is full and turgid in live specimens); subgenital plate of male elongate and rectangular, 4.2–5.6 (5.6) mm long, and 1.7–2.7 (2.2) mm wide, concave ventrally, deepest in middle ( Fig 5 View FIGURE 5. a d, e), ventral face with strong lateral carinae straight until apex with apical portions incurved with one small apical tooth on each side, median ridge of ventral face straight throughout, anterior margin acute, posterior margin broadly emarginate between the incurved ends of the lateral carinae ( Fig 5 View FIGURE 5. a d, e); subgenital plate of female ( Fig 6a View FIGURE 6. a ) triangular, 2.1–3.6 mm long and 2.5–3.0 mm wide, broad across anterior margin and tapering to a rounded point on posterior margin, anterior lateral lobes rounded, median 1/3 elevated from anterior to posterior margins, with a longitudinal central groove throughout, deepest in the posterior portion, anterior margin down curved ventrally, posterior margin not curved, posterior apex narrowly emarginated forming a V-shaped indention ( Fig 6 View FIGURE 6. a b); female ovipositor shorter than hind femur, 8.7–12.4 mm long, at base, and 2.2–2.7 mm wide at base, laterally flattened, and tapering to apical point, upturned throughout, but abruptly upturned about 2/3 the distance from the base ( Fig 6 View FIGURE 6. a c), row of 15–38 small regularly spaced teeth on dorsal margin up upper valve beginning at mid-distance from base to the apex, row of 12–27 teeth on ventral margins of upper valves beginning about 3/4 the distance from base to, row of 13–21 teeth on ventral margin of lower valves beginning about 4/5 the distance from the base to the apex, no teeth on dorsal surface of lower valves, both upper and lower valves with a tooth at the apex ( Fig 6 View FIGURE 6. a c).
Coloration ( Figs 1 View FIGURE 1 , 2 View FIGURE 2. a ). Overall coloration varies from individuals that are largely black dorsally, to individuals that are largely brown to tan dorsally, metazona always reddish-brown; head uniformly black to brown dorsally above eyes and antennae, except for light brown patches on either side of the posterior margin of the occiput, those patches much larger and linear, extending laterally along the vertex to the posterior margin of the occiput, pale individuals have a large tan patch posterior to the eye, below the eye becoming dark brown to light brown with scattered tan to whitish dots on the frons and gena, lighter brown to tan on the clypeus and labrum, also with scattered tan or whitish dots; eye black to dark brown with lighter mottling; palps tan; scape and pedicel of antenna tan, flagellum dark brown to black, with repeated banding pattern of single or double whitish segments at intervals of about every 5–7 segments on the basal half of the flagellum, to intervals of 8–10 segments on the apical half of the flagellum; pronotum black to brown on dorsum of prozona, side of prozona also black to brown dorsally, with black patch along the lateral shoulder, posterior margin and lateral lobe of prozona abruptly becoming tan to whitish and extending up to the anterior base of the metazonal lateral carina creating a triangular light mark, metazona uniform dark to light reddish-brown dorsally throughout, anterior portion of metazona lateral lobe often tan to whitish near anterior ventral margin, and often black on lateral lobe from lateral margin mid-way to the lateral carina; remainder of thorax black to brown with considerable tan to whitish maculations, sternal plates light brown to tan with some lateral brown mottling and considerable mottling of whitish dots; wing pads uniform pale brown to tan; fore and middle legs generally a mix black or brown with considerable mottling of tan to whitish dots, especially on the femora, spines and teeth pale brown or tan basally, and black or brown apically; hind femora dorsal surface black to brown, mottled with tan to whitish dots, a heavy concentration of light mottling forming a large tan to whitish patch across the lateral and dorsal surface about 1/5th the distance from the base, and another light band on the narrowest portion of the femur about ¼th the distance below the apex, the size of those light patches much larger in pale individuals, ventral surface of hind femora tan, hind tibia uniform brown to tan, usually with dark brown or black maculations, spines and teeth, pale brown or tan basally, and black or brown apically; abdomen black to brown dorsally, tergites 5–8 with patterns of tan to whitish mottling only in dark individuals, but usually forming quadrate to ovoid tan or whitish medio-lateral markings on tergites 5 and 6, the largest of which occurs on tergite 6, in pale individuals the markings appear on the dorsal surfaces of all tergites, lateral bases of tergites with considerable mottling of whitish dots; sternites uniform reddish brown lightly patterned with whitish dots; supraanal and subgenital plates of males and females usually uniform reddish brown to light brown, occasionally lightly patterned with whitish dots, paraprocts of male uniform reddish brown, female ovipositor usually reddish brown, varying to tan or pale brown, lightly streaked with whitish markings, basally black or brown, teeth black or brown.
Measurements. See Table 2 View TABLE 2 for values of all morphological characters measured, including the minimum, maximum and average, for both males and females. See Appendix 1 for an illustrate guide as to where measurements were taken on specimens.
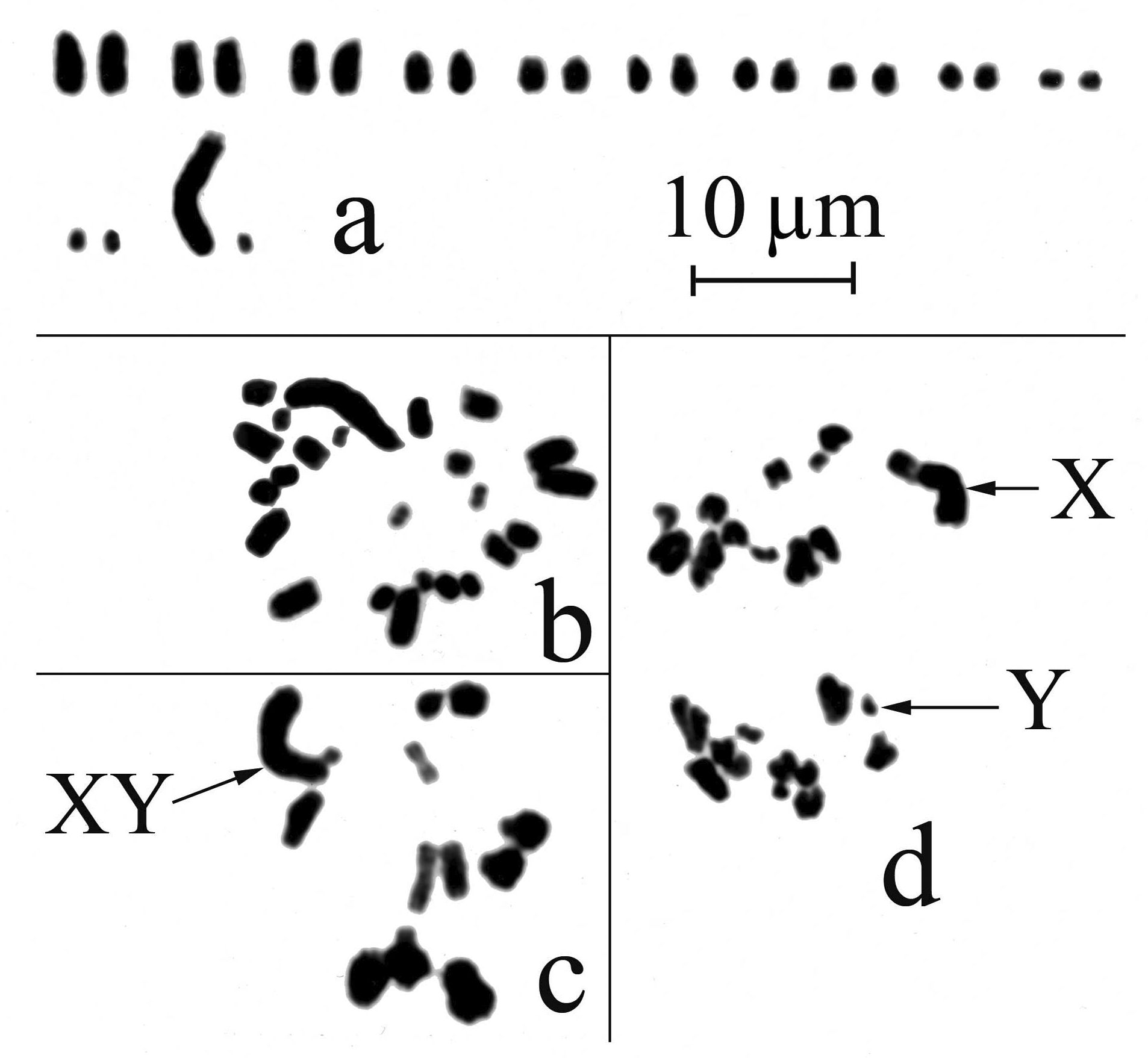
Karyotype ( Fig 7 View FIGURE 7 ). 2n3 =24(22t + XtYt), consisting of 11 pairs of medium to small telocentric autosomes, a very large telocentric X chromosome and a small telocentric Y chromosome ( Figs 7 View FIGURE 7 a [idiogram], b [mitosis]). First metaphase shows 11 autosomal bivalents and a consistently associated large X and small Y ( Fig 7 View FIGURE 7 c), and at first anaphase with 11 autosome halves with X at top pole and 11 autosome halves and Y at the bottom pole ( Fig 7 View FIGURE 7 d). Seven males analyzed.
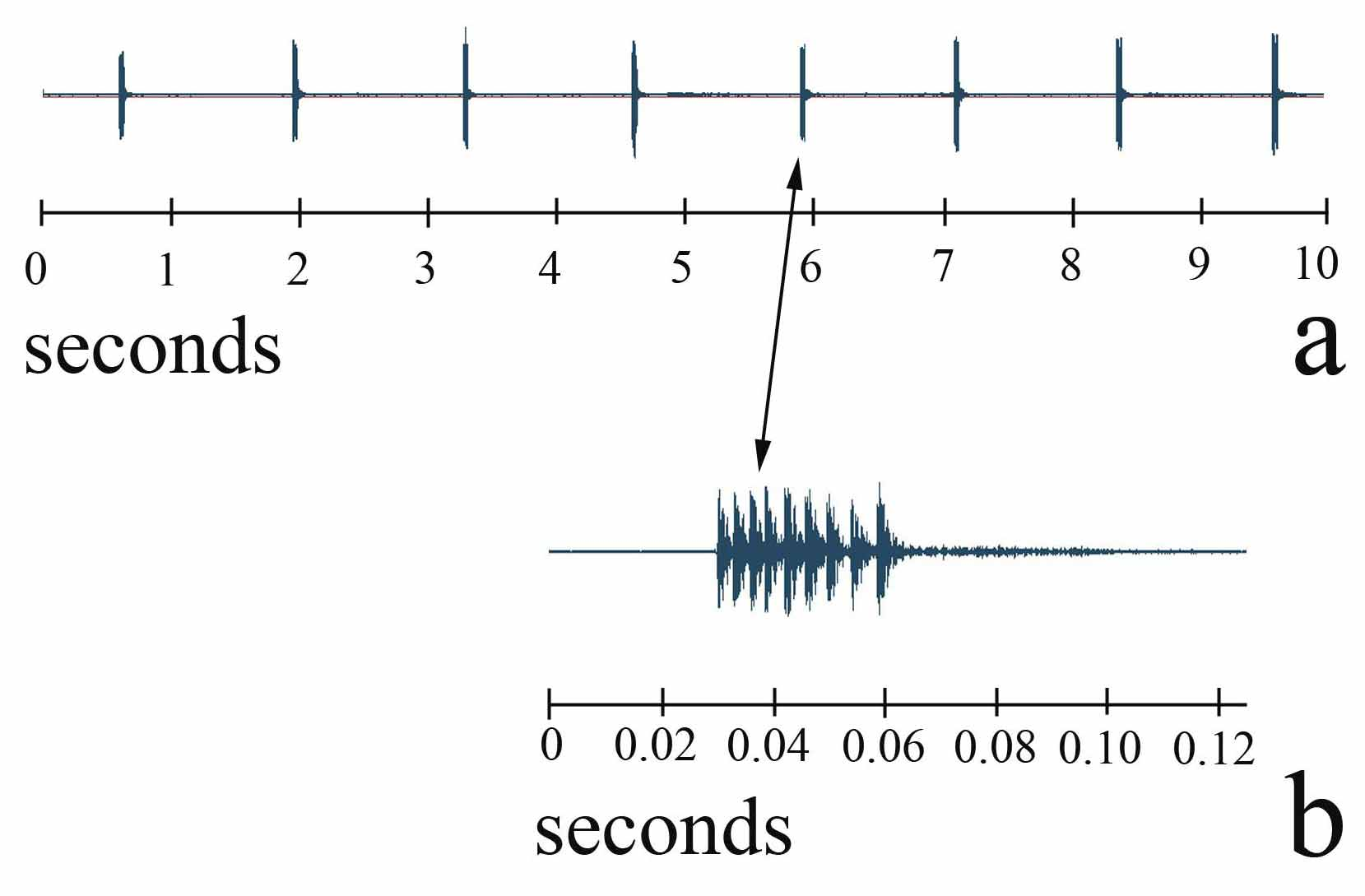
Calling song ( Fig 8 View FIGURE 8 ). The calling song of P. jacintotopos consists of single “swick” pulses with intervals of between 0.8 and 1.0 seconds between pulses at temperatures of 23–25 C ( Fig 8 View FIGURE 8 a). In the field these swicks are usually delivered much more slowly since the ambient temperature is much lower. Fig 8 View FIGURE 8 b displays a 40X magnification of the 5th pulse showing 9 units, which probably represent individual tooth strikes. These tooth strikes are produced at an average rate of 286 per second. The number of units in the 8 swicks shown in Fig 8 View FIGURE 8 ranged from 7 to 10 with unit rates varying from 231 to 286 per second. If these units are tooth strikes, that is surprisingly few teeth used considering that males average 91 teeth in the file ( Table 2 View TABLE 2 ). We believe that a combination of pronotal shape and “broadcasting properties,” combined with infrequent pulses, results in singing males being extremely difficult to triangulate in the field.
Natural history. P. jacintotopos appears to have one generation per year with adults known from August through January. Phymonotus appears to be arboreal and closely associated with conifer trees, especially Jeffery ( Pinus jefferyi ) and Ponderosa ( Pinus ponderosa ) pines in montane mixed-conifer forests composed of Jeffery and ponderosa pine, white fir ( Abies concolor ) and incense cedar ( Libocedrus decurrens ). The body coloration and patterns of Phymonotus provides camouflage on the bark of Jeffery pine ( Fig 1 View FIGURE 1 ) and other conifer trees. The type series was collected mainly from ground covering Vinca (periwinkle) in a landscaped residential yard beneath a Jeffery pine forest over-story. During a second visit to the type locality, many individuals were heard calling from the pine trees, and none were found near the ground. In natural situations individuals have been found almost exclusively in tree canopies and on tree trunks high above the ground where their mottled dark reddish-brown coloration provides good camouflage. The stout, toothed ovipositor of the female may be used to oviposit into bark, as Rentz and Birchim (1968) suggest for Neduba and Agalothorax . E. R. Tinkham (field notes) kept live adults in captivity and noted that they readily fed on the needles of both Jeffery and ponderosa pine, and on the foliage of incense cedar. P. jacintotopos appears to be adapted to cool temperatures as males have been heard calling on cold nights, and have been collected in early January while snow was on the ground and ambient temperatures near freezing (E. R. Tinkham, field notes). Males may also call during the day late in the season when night temperatures are apparently too cold. We found P. jacintotopos to be common in Fern Valley, where many individual males could be heard calling from trees at night.
Phymonotus View in CoL males may produce thoracic gland secretions ( Gwynne 2001) as a nuptial meal for females prior to and during mating. D. C. F. Rentz (personal communication April, 2011) informed us that E. R. Tinkham reported to him that males produced a fluid from under the tegmina during courtship, and that females fed upon the fluid prior to mating. We did not observe courtship, mating, or thoracic gland secretions in Phymonotus View in CoL , but we did find evidence of dried fluid on the dorsal postnotal segment and first abdominal tergite of many pinned male specimens. We did not find any evidence of visible gland openings, but the dorsal postnotal segment of Phymonotus View in CoL males does possess shallow lateral depressions that may serve to retain fluid. Thoracic gland secretions might be extruded from membranes under the dorsal postnotal segment. Such thoracic gland secretions are known to be secreted from the metanotal gland by male tree crickets ( Oecanthus ) ( Walker and Gurney 1967), and the secretions are known to increase insemination and to extend the reproductive life-span of female black-horned tree crickets ( Oecanthus nigricornis F. Walker ) ( Brown 1997). We did not find evidence that females chew on the male wings, also an indication of nuptial meals offered by male hump-winged crickets ( Cyphoderris View in CoL ) to females during courtship and mating ( Gwynne 2001). Examination of numerous pinned specimens of Agalothorax View in CoL and Neduba View in CoL males revealed no indication of dried thoracic gland secretions as found in Phymonotus View in CoL , but both genera do possess the same lateral depressions in the dorsal postnotal segment as we found in Phymonotus View in CoL . Further research should be conducted to determine the presence and nature of thoracic gland secretions in Phymonotus View in CoL , a feature unknown in any other Nedubini View in CoL or Tettigoniini View in CoL world-wide.
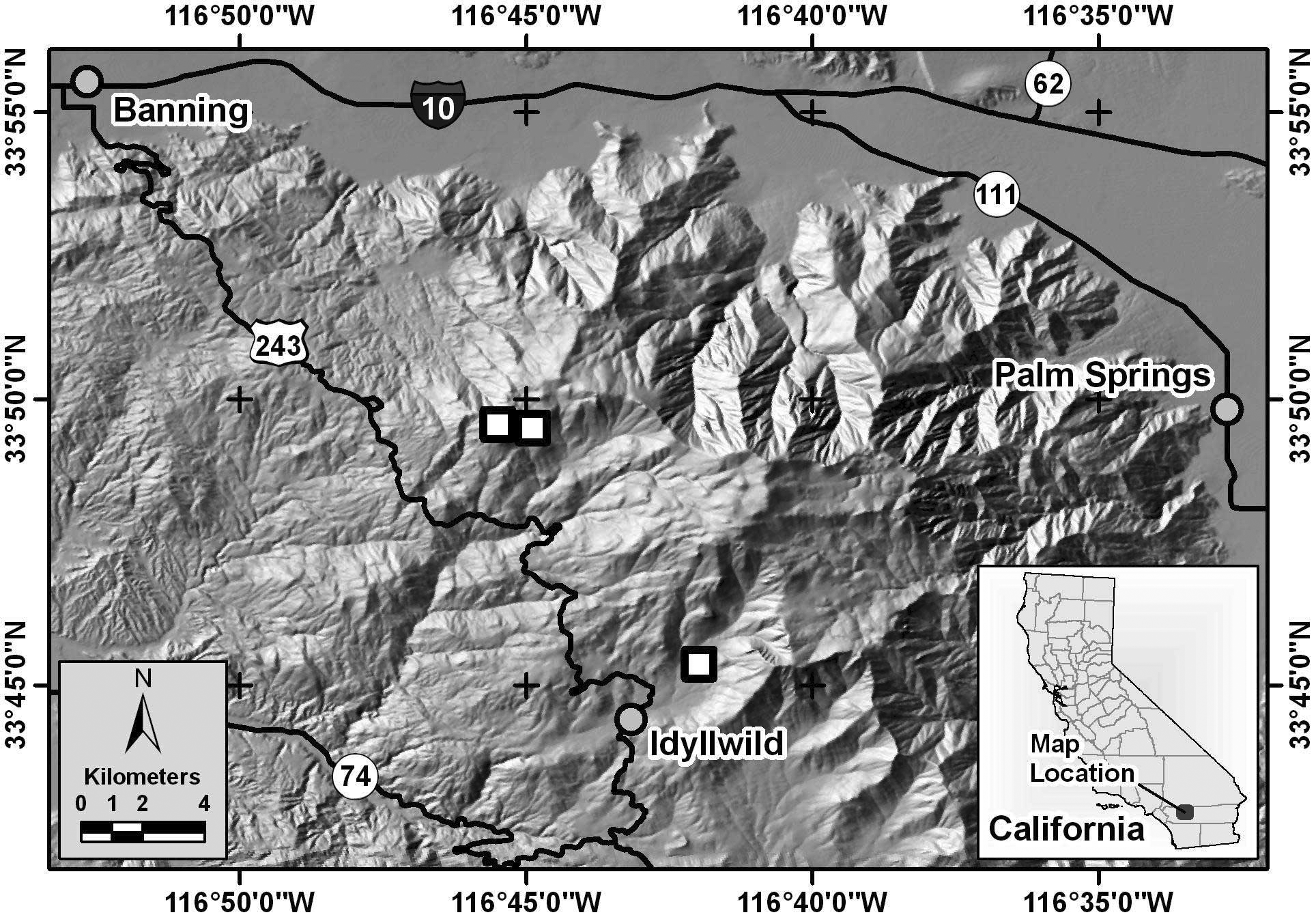
Distribution. Phymonotus is known only from the San Jacinto Mountains, Riverside County, California ( Fig 3 View FIGURE 3 ). Phymonotus has been found only in the Fern Valley and Boulder Basin Campground areas on the west side of the San Jacinto Mountains near the town of Idyllwild, from approximately 1,500 m to 2,500 m elevation. Despite extensive field surveys by us in the nearby San Bernardino, San Gabriel, and Tehachapi Mountains, which also support conifer forests and related Agalothorax and Neduba species, no Phymonotus were heard. Other related Agalothorax and Neduba taxa occur throughout southern California at lower elevations in chaparral, pinyon/juniper woodland and desert shrub, especially on Joshua tree ( Yucca brevicauda ).
TABLE 1. Comparative character states for Neduba, Agalothorax, and Phymonotus that differentiate genera of North Ameri-
| Lateral lobes* of prono- tum | both | one | one | two |
|---|---|---|---|---|
| Metazona* dorsal shape | both | flat | flat | dome |
| Metazona * posterior dorsal margin | both | acute | acute | concave |
| Supra-anal* plate shape | male | quadrate | oval | hour-glass |
| Titillator arms | male | robust, not diverging | sinuous, diverging | sinuous, diverging |
| Base of* Titillator | male | sclerotized | sclerotized | not sclerotized |
| Dorsal sclerite of titilla- tor | male | absent | absent | present |
| Ventral sclerite* of titil- lator | male | present | absent, but teeth present on ventral lobe | absent and no teeth on ven- tral lobe |
| Calling song* | male | continuous, with several pulse types | single pulses with short intervals | single pulses with long intervals |
| Subgenital plate | female | no spiniform appendages | Spiniform appendages | no spiniform appendages |
| Subgenital* plate | female | no apical indentation | no apical indentation | Apical indentation |
TABLE 2. Phymonotus jancintotopos gen. et sp. nov. morphological measurements in millimeters, measured from 15 female and 24 male adult paratype specimens, and from the male holotype. See species’ description text for holotype measurements in parentheses. See Appendix 1 for details on how measurements were taken.
| Morphological Feature Body Length Head Width Head Length | Male Average 15.74 4.52 2.24 | Maximum 18.20 5.48 2.82 | Minimum 13.69 3.90 1.74 | Female Average 18.48 5.13 2.67 | Average 20.88 5.73 3.32 | Minimum 13.53 3.98 1.83 |
|---|---|---|---|---|---|---|
| Head Vertex Width Eye Width | 0.58 1.08 | 0.67 1.18 | 0.50 0.97 | 0.68 1.19 | 0.84 1.39 | 0.46 1.01 |
| Eye Height Inter-eye Distance Prozona Length Prozona Dorsal Width Prozona Ventral Width Prozona Depth | 1.24 2.53 2.49 2.01 5.62 2.61 | 1.34 2.82 2.91 2.49 6.47 3.07 | 1.13 2.24 2.07 1.58 4.81 2.07 | 1.37 2.86 2.91 2.27 6.22 2.98 | 1.55 3.24 3.57 2.49 7.06 3.57 | 1.18 2.24 2.32 1.83 4.90 2.49 |
| Metazona Length Metazona Dorsal Width Metazona Ventral Width Metazona Depth Subgenital Plate Length Subgenital Plate Width | 4.37 5.80 6.27 4.03 5.10 2.21 | 5.48 6.81 7.05 4.90 5.64 2.74 | 2.74 4.40 5.64 3.24 4.23 1.66 | 3.43 5.77 6.65 3.04 3.08 2.80 | 3.98 6.56 7.39 3.65 3.57 2.99 | 2.66 4.57 5.31 2.24 2.08 2.49 |
| Hind Femur Length Hind Femur Width Wing File Length | 12.75 2.61 3.44 | 14.53 3.24 3.90 | 10.02 2.08 3.10 | 14.33 2.91 NA4 | 16.03 3.24 NA | 10.69 2.08 NA |
| Wing File Tooth Count Ovipositor Length | 90.9 NA | 111.0 NA | 81.0 NA | NA 10.84 | NA 12.36 | NA 8.68 |
| Ovipositor Width Ovipositor Teeth Dorsal1 Ovipositor Teeth Middle2 Ovipositor Teeth Ventral3 | NA NA NA NA | NA NA NA NA | NA NA NA NA | 2.46 26.2 20.5 16.0 | 2.74 38.0 27.0 21.0 | 2.16 15.0 12.0 13.0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.