Clathria (Thalysias) curacaoensis Arndt, 1927
|
publication ID |
https://doi.org/10.11646/zootaxa.3835.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:E3F3FD5C-E526-4A66-911F-0FF5D692AAA8 |
|
DOI |
https://doi.org/10.5281/zenodo.6130511 |
|
persistent identifier |
https://treatment.plazi.org/id/336C1F6A-D75A-E725-4AD6-FCF8FDADFD3E |
|
treatment provided by |
Plazi |
|
scientific name |
Clathria (Thalysias) curacaoensis Arndt, 1927 |
| status |
|
Clathria (Thalysias) curacaoensis Arndt, 1927 View in CoL
Figures 4 View FIGURE 4 , 5 View FIGURE 5 , 6 View FIGURE 6 ; plate 1 figures C, D, E
Clathria copiosa View in CoL var. curacaoensis Arndt, 1927: 148 View in CoL , pl. I fig. 3, text-fig. 9.
Aulospongus schoenus de Laubenfels, 1936: 100, pl. 13 fig. 3.
Thalysias schoenus View in CoL ; Simpson 1968: 56, pls. 13–14, text-fig. 5 (also fide van Soest 1984); Randall and Hartman 1968: 223 (also fide Hooper 1996: 411); Alcolado 1980: 4 (also fide van Soest 1984).
Rhaphidophlus schoenus View in CoL ; van Soest 1984: 112, pl 8 fig. 1–4, fig. 44.
Non: Rhaphidophlus schoenus ; González Calderón 1992: 319 (ecology); Zea 1993: 82, 88 (ecology) [both= Clathria (Thalysias) venosa ( Alcolado, 1984) View in CoL ]; Chen and Mok 1993: 278 ( fide Hooper,1996: 411 as a possible misidentification from Taiwan).
Clathria (Thalysias) schoenus View in CoL ; Hooper 1996: 411 (synonymy and distribution); Lehnert and van Soest 1998: 87; Díaz et al. 2005: 471; Collin et al. 2005: 655, fig.; Freeman et al. 2007: 323; Zea and Díaz-Sánchez 2011: 219 (ecology); Valderrama and Zea 2013, table 2.
Clathria (Thalysias) View in CoL ? schoenus View in CoL ; Zea et al. 2009.
Clathria schoenus View in CoL ; Rützler et al. 2000: 235; Zea 2001, addendum Table 1d (ecology); Alcolado 2002: 64; Rützler et al. 2009: 299; Alcolado and Busutil 2012: 69.? Hajdu et al. 2011: 133, figs.
Rhaphidophlus raraechelae View in CoL van Soest, 1984: 116, pl. 8 fig. 5, fig. 46;? Pulitzler-Finali 1986: 151; Zea 1993: 88.
Clathria (Thalysias) raraechelae View in CoL ; Lehnert and van Soest 1998: 87.
Clathria raraechelae View in CoL ; Humann et al. 2013: 27, fig.
Rhaphidophlus juniperinus ; Reyes and Campos 1992, Table 2 (ecology).
Non: Microciona microchela Hechtel, 1965: 41 , fig. 7 [synonymy suggested by van Soest 1984: 112; = Clathria (Thalysias) venosa ( Alcolado, 1984) View in CoL ].
Rhaphidophlus venosus ;? Kobluk and van Soest 1989: 1216;? Gammill 1998: 91, photo 89.
Non: Clathria (Thalysias) venosa ( Alcolado, 1984) View in CoL (a valid species; also erroneously synonymized with Rhaphidophlus raraechelae by Hooper 1996).
Non: Clathria (Thalysias) hechteli Hooper, 1996 View in CoL [from suggested possible synonymy with Rhaphidophlus schoenus sensu van Soest, 1984 by Hooper 1996), = Clathria (Thalysias) venosa ( Alcolado, 1984) View in CoL ].
Microciona View in CoL sp. 4; Sánchez 1984: 55, fig. 6.18 B.
Microciona View in CoL sp.; Díaz et al. 1985: 38, fig. 10d–f.
Material examined. Holotype, ZMA POR.726, Curaçao, Spaanse Water, encrusted on coral Porites porites, coll. C.J. van der Horst, May 1920; Holotype of Aulospongus schoenus , USNM 22404 (tissue slide INV-POR 1215), East of Loggerhead Key, Dry Tortugas, Florida, 17 m depth, coll. M.W. de Laubenfels, 22 Jun. 1932; Holotype of Rhaphidophlus raraechelae , ZMA POR. 4874 (spicule slides INV-POR 1213), Curaçao, Boei 0, 23– 35 m, on dead coral, coll. R.W.M. van Soest, 23 Dec. 1980 [and tissue slides of paratype ZMA POR. 4875, and specimens ZMA POR. 3604 and 4877, also from Curaçao). Santa Marta: ICN-MHN(Po) 259 ( 20 May 1982), INV-POR 1211 ( 17 Feb. 2011), Bahía de Nenguange, on mangrove root, 0.5 m; INV-POR 1203, Bahía de Santa Marta, Punta de Betín, dock piling, on bivalve Arca imbricata , 9 m, 24 Nov. 1981; INV-POR 1204 ( 11 mar. 1988), 1205 ( 6 Apr. 1988), 1206 ( 21 Dec. 1993), Bahía de Santa Marta, Morro, overhanging rock walls, 4– 5 m. Cartagena, Islas del Rosario: INV-POR 1207 (on rubble at the shore, 0.8 m, 9 Mar. 1980), 1208 ( Thalassia bed, 2 Aug. 1980), 1209 ( Thalassia bed, 1 m, 29 Jan. 1983), south of Isla Grande; INV-POR 1210, Pajarales, Pretelt island, on standing dead Acropora cervicornis , 6 m, 31 Jan. 1983: INV-POR 1216, Isla Tesoro, north slope, on black coral, 24 m, coll. K. Dunlap, 2 Aug. 1980. Venezuela: INV-POR 1212, Puerto Cabello, Isla Larga, on Arca shell on a sunken ship, 5 m, coll. M.A. Tálamo, 1990. Material from the Roncador Bank lagoon (SW Caribbean, Colombia) and Bocas del Toro fringing reefs, Panama was also examined.
Shape, color and consistency. Thinly encrusting, spreading irregularly over the substratum for several tens of centimeters. There are ramose specimens with anastomosing branches, up to 6–7 mm in thickness, rising 5–10 cm; encrusting specimens in calm environments (e.g., mangrove stilt roots) often grow branches. Surface even, smooth, shiny, with a conspicuous vein pattern of the canal system and oscules clearly visible to the naked eye; exhalant canals are about 1–2 mm in maximum width, oscules up to 4–5 mm in diameter, 0.5–2 cm apart. External color yellowish, interior orange; on close-up view, there is a transparent membrane overlying a subectosomal, orange yellow, net-like grainy structure; membrane over canals is also transparent, with lines of white-grayish dots, through which the orange color of the choanosomal tissue is visible. Consistency soft, slightly slimy to the touch; ramose specimens with soft skin but internal fibers are tougher, somewhat elastic.
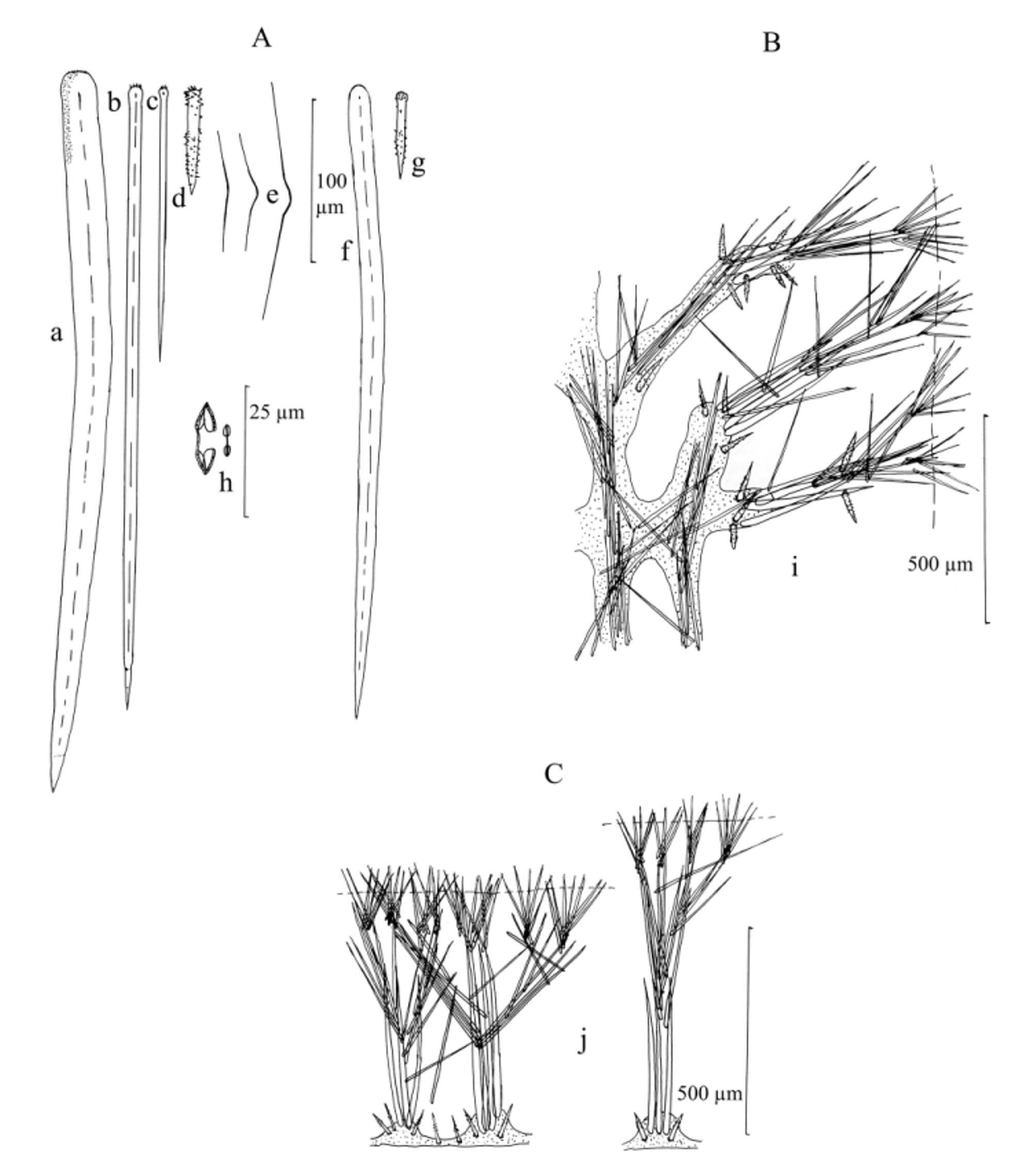
Skeleton. Ectosome as thin pinacoderm supported by brushes of small tylostyles. In encrusting portions, the choanosomal skeleton is made of groups of 3–5 erect principal styles, rising from a basal plate of spongin, 100–150 µm apart, surrounded at the base by echinating accessory acanthostyles; at their medial and apical portions they are continued further upwards by tracts of 1–10 styles and/or auxiliary subtylostyles. Depending on the thickness of the specimen, these tracts end in the ectosomal brushes of small auxiliary subtylostyles, or continue branching upright and sidewise. In branches there is a choanosomal fibrous network of ascending and interconnecting spicule tracts, 25–100 µm in thickness, more or less surrounded by spongin; these tracts are composed of 1–5 thick styles and/or 3–10 subtylostyles in a plumose arrangement; the styles are especially present at branching points of the fibrous network, where echinating acanthostyles surround their basal parts; acanthostyles are rare outside these branching points, and if they occur elsewhere in the fibers, they are usually positioned in parallel to the spicule tracts. From the central fiber network, tracts and fibers diverge towards the surface every 150–430 µm, ending in ectosomal brushes in a fashion like the encrusting areas. Spicules (Table 1): (1) Basal and coring principal styles, slightly curved, with smooth heads (sometimes microspined) and slightly telescopic ends, 133– 338.2 –489 µm by 4.8– 13.6 –24.7 µm; specimens from Cartagena have larger and thicker styles than specimens from Santa Marta (means 349.6 µm by 17.6 µm vs. 326.8 µm by 9.5 µm, respectively, Table 1). (2) Straight auxiliary subtylostyles, with small, frequently microspined heads and slightly telescopic ends; these occur in two (possibly three) size categories by spatial location: large, choanosomal and subectosomal ones, 219– 304.5 –447 µm by 2.9– 5.5 –10.9 µm, and small, ectosomal ones, 100– 138.7 –200 µm by 1.9– 2.9 –5.7 µm; the larger category is also larger at Cartagena (mean 328.7 µm by 6.2 µm) than at Santa Marta (mean 280.3 µm by 4.8 µm) (Table 1). (3) Echinating accessory acanthostyles with small, numerous spines located mainly at the head and at the distal half of the shaft, leaving the proximal half mostly smooth, 47– 57.7 –70 µm by 4.5– 6.1 –9.0 µm. (4) Thin toxa, wing-shaped, accolade and raphidiform, 43– 125.7 –176 µm. (5) Palmate isochelae in two size categories: rare, large ones, 12.7– 13.5 –15 µm; more abundant, tiny ones, often twisted-contorted, 4.6–5.9 µm.
Distribution and ecology. Georgia ( Freeman et al. 2007), Bahamas ( Simpson 1968; Zea et al. 2009); east coast of Florida ( Simpson 1968, de Laubenfels 1936), NW Gulf of Mexico ( Rützler et al. 2009), Cuba ( Alcolado 1980, 2002), US Virgin Island and Puerto Rico ( Randall & Hartman 1968; van Soest 1984), Jamaica (Lehnert & van Soest 1998), Venezuela (Puerto Cabello; Morrocoy, Díaz et al. 1985), Curaçao ( Arndt 1927, van Soest 1984), Bonaire (van Soest 1984), Colombia [Santa Marta (also Sánchez 1984; Reyes & Campos 1992; Zea 1993), Cartagena (also Zea & Díaz-Sánchez 2011), Urabá ( Valderrama & Zea 2013), Albuquerque Cays ( Zea 2001), Courtown Cays (S.Z. unpublished observations), Old Providence (S.Z. unplublished observations), Roncador Bank], Panama (Bocas del Toro, Díaz et al. 2005; Collin et al. 2005), Belize ( Rützler et al. 2000). This species is relatively common encrusting overhanging and shadowed hard substrata (rocks, sides and undersides of corals, dock pilings, sunken ships and mangrove stilt roots). Ramose specimens are more common on rubble and sand within sea-grass and algal beds. In sheltered locations it may occur in depths as shallow as half a meter; in open rocky and reef locations, from 3–4 m down to the reef base ( 25–35 m).
Table 1. Spicule types and sizes of Clathria (Thalysias) species described here. Data are min.– mean –max length x width (when relevant) in Μm (from about 25 spicules of one to several specimens).
Auxiliary subtylostyles Toxa Chelae
Species Principalstyles ___________________________ Accessory ________________________________________ ________________________
acanthostyles
Large Small Oxeote Mid-size Small Large Small (choanosomal) (ectosomal)
Remarks. After revision of the types, we concur with van Soest (1984) in the synonymy of Aulospongus schoenus de Laubenfels, 1936 and Clathria copiosa var curacaoensis Arndt, 1927 (see Figure 6 View FIGURE 6 ). Despite curacaoensis being an older available name, van Soest (1984) favored schoenus as valid for being more established. From a suggestion of R.W.M. van Soest himself ( in litt.), we have here applied the rule of priority and advanced curacaoensis as the valid species name (see also a note in van Soest et al., 2013: 322). On the other hand, we also found that the type (and three more specimens available at ZMA) of Rhaphidophlus raraechelae van Soest, 1984 also belongs to C. curacaoensis ; we have thus here included the former species as junior synonym. There has been a great deal of confusion in the names used for two species that are herein known as Clathria (Thalysias) curacaoensis and Clathria (Thalysias) venosa ( Alcolado, 1984) (see description below). C. venosa forms large encrustations, with a vein-pattern of the canal system even more conspicuous than that of C. curacaoensis , and with grayish or pinkish external color and bright orange internal color, as opposed to a transparent skin and an orange-yellow internal color in C. curacaoensis . The two species are also distinguished by their spicules, C. venosa having principal styles with irregularly verrucose heads, while in C. curacaoensis these are smooth, besides other differences in toxas (long oxeote and very small ones only present in C. venosa ). In addition, C. venosa seldom or never grows branches. These two species were also clearly distinguished by Gómez (2014), although she used the name C. raraechelae for C. curacaoensis . By examination of material or from descriptions and photos we have sorted several records of schoenus , raraechelae and venosa - venosus (and also of juniperinus ) between C.
curacaoensis View in CoL and C. venosa View in CoL ( Table 2). In fact, simultaneously with the publication of the original description of C. venosa View in CoL , van Soest (1984) synonymized Microciona microchela Hechtel, 1965 with A. schoenus , but M. microchela turned out to be conspecific with C. venosa View in CoL (see below). On the other hand, van Soest (1984) mentioned grayish specimens in R. raraechelae , indicating that he may have included characteristics of C. venosa View in CoL within his scope of R. raraechelae . This earlier confusion probably led Kobluk and van Soest (1989) to synonymize R. raraechelae with R. venosus , and indeed led González Calderon (1992) and Zea (1993) to use the name R. schoenus for C. venosa View in CoL , and R. raraechelae for the then considered valid name R. schoenus . It may also have led Hooper (1996) to synonymize R. raraechelae with C. venosa View in CoL , and R. schoenus sensu van Soest with M. microchela (using a new name, C. hechteli View in CoL , because there was a homonym from the NE Atlantic). Gómez (2014) mentions undescribed material close to C. schoenus from Yucatan. In addition, there are records of R. raraechelae such as that of Pulitzer- Finali (1986), and of R. venosus such as those of Gammill (1998) and Kobluk and van Soest (1989) that still need to be clarified. Clathria schoenus View in CoL described by Hajdu et al. (2011) from Bahía, Brazil, does not look externally like C. curacaoensis View in CoL and although there is also a small category of chelae, the toxa do not match, and specimens representing the species look thicker. We also established that the Santa Marta record of Rhaphidophlus juniperinus by Reyes & Campos (1992) belongs to C. curacaoensis View in CoL by re-examination of the material (INV-POR 428). Descriptions and illustrations of Microciona sp. 4 by Sánchez (1984, Santa Marta), and Díaz et al. (1985, Venezuela) match C. curacaoensis View in CoL .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Microcionina |
|
Family |
|
|
Genus |
|
|
SubGenus |
Thalysias |
Clathria (Thalysias) curacaoensis Arndt, 1927
| Zea, Sven, Rodríguez, Angélica & Martínez, Ana María 2014 |
Clathria raraechelae
| Humann 2013: 27 |
Clathria schoenus
| Alcolado 2012: 69 |
| Rutzler 2009: 299 |
| Alcolado 2002: 64 |
| Rutzler 2000: 235 |
Clathria (Thalysias) raraechelae
| Soest 1998: 87 |
Clathria (Thalysias) schoenus
| Zea 2011: 219 |
| Freeman 2007: 323 |
| Collin 2005: 655 |
| Soest 1998: 87 |
| Hooper 1996: 411 |
Rhaphidophlus venosus
| Soest 1989: 1216 |
Microciona
| Diaz 1985: 38 |
Rhaphidophlus schoenus
| Soest 1984: 112 |
Rhaphidophlus raraechelae
| Zea 1993: 88 |
| Soest 1984: 116 |
Microciona
| Sanchez 1984: 55 |
Thalysias schoenus
| Hooper 1996: 411 |
| Alcolado 1980: 4 |
| Simpson 1968: 56 |
| Randall 1968: 223 |
Aulospongus schoenus
| Laubenfels 1936: 100 |
Clathria copiosa
| Arndt 1927: 148 |