Philander pebas, Voss & Díaz-Nieto & Jansa, 2018
|
publication ID |
https://doi.org/ 10.1206/3891.1 |
|
persistent identifier |
https://treatment.plazi.org/id/36452319-FF90-FFD9-11D8-FA93FC52FAB2 |
|
treatment provided by |
Carolina |
|
scientific name |
Philander pebas |
| status |
sp. nov. |
Philander pebas , new species
TYPE MATERIAL: The holotype, MVZ 190343, consists of the skin, skull, and frozen tissues of an adult male collected by J.L. Patton (original number 15395) on 1 September 1991 at Igarapé Nova Empresa , on the left bank of the Rio Juruá , Amazonas, Brazil (6°48´S, 70°44´W). A complete (1149 bp) cytochrome- b sequence that we obtained from this specimen is archived in GenBank with accession number MG491956 View Materials GoogleMaps .
DISTRIBUTION AND SYMPATRY: Sequenced specimens and other referred material of Philander pebas are from eastern Ecuador, eastern Peru, and Amazonian Brazil ( fig. 9 View FIG ). Based on specimens we examined, P. pebas occurs sympatrically with P. andersoni in northeastern Peru (e.g., near Iquitos, in Loreto department) and with P. canus and P. mcilhennyi in southeastern Peru (e.g., at Balta in Ucayali department).
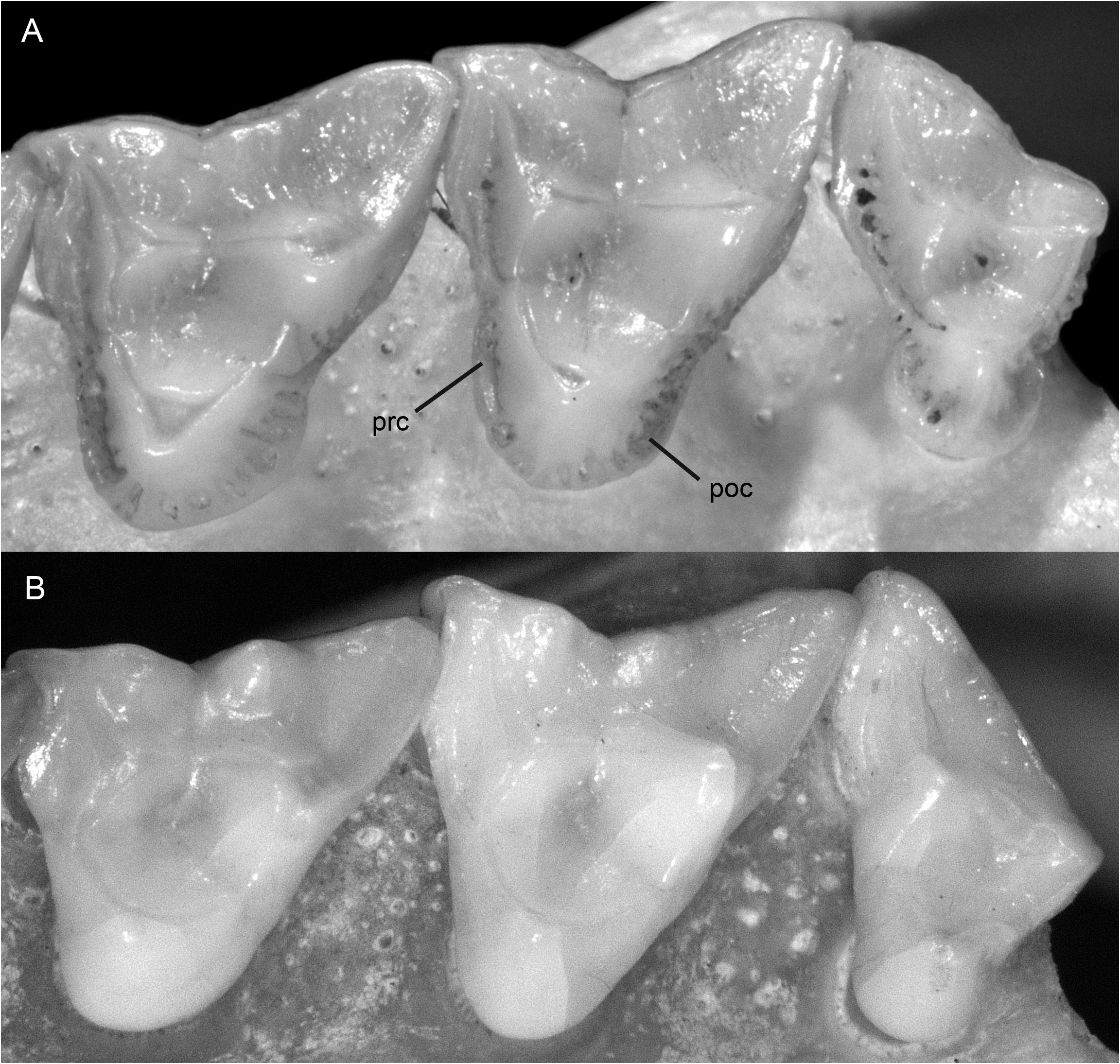
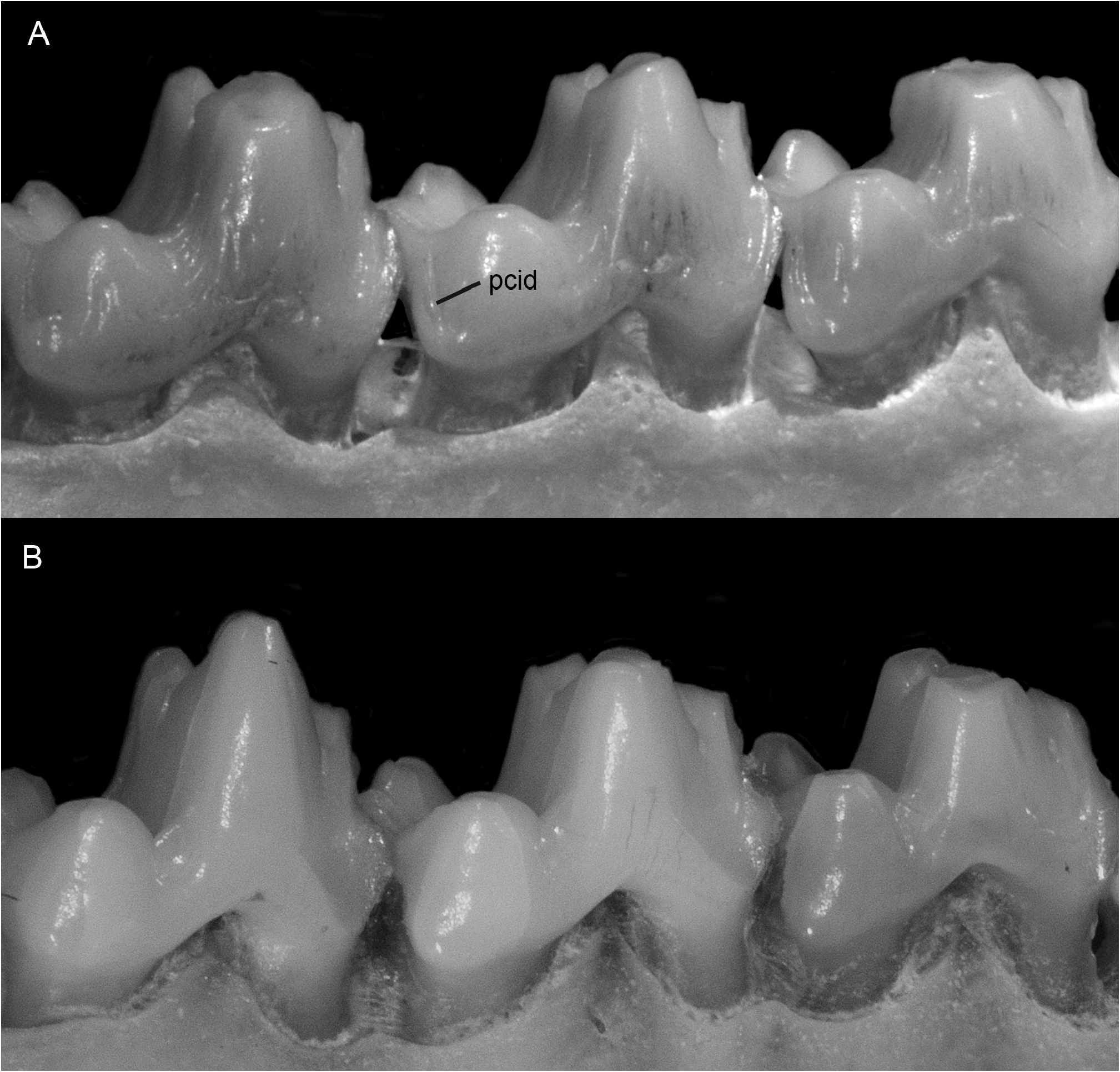
DESCRIPTION: Dorsal pelage very short (usually ≤ 12 mm) and uniformly grayish (sometimes darker middorsally than on the flanks but never with a distinct middorsal blackish stripe; fig. 10 View FIG ); fur of crown (between the ears) grizzled-grayish, often quite dark but apparently never clear black (at least some hairs frosted, with pale tips); pale preauricular spot absent or indistinct; ventral fur mostly gray-based ( fig. 11 View FIG ), often self-cream or -buffy in the inguinal region but apparently never continuously self-colored along the abdominal and thoracic midline; pinnae sometimes entirely blackish but often indistinctly paler basally; hind feet often with dark metatarsals and pale digits, but not blackish or with distinctly blackish markings; scaly part of tail usually <¼ white distally. Nasal bones neither very short nor unusually elongated (about 47% of condylobasal length on average), sometimes extending posteriorly to (but apparently never between) postorbital processes. Unworn third upper premolar (P3) with complete labial cingulum; crown length of upper molar series 13.8 ± 0.5 mm (sexes combined; observed range 12.7–15.1 mm, N = 50); unworn molar enamel distinctly crenulated, especially on lingual surfaces of protocones ( fig. 19A View FIG ); pre- and postcingula usually present on one or more upper molars (more frequently retained on M4 than on M 1–3 in older specimens with worn teeth; fig. 19A View FIG ); posterior cingulids apparently always present on one or more lower molars ( fig. 20A View FIG ).
PHYLOGEOGRAPHY AND GEOGRAPHIC VARIATION: Some phylogeographic structure is apparent among the 14 haplotypes that we assign to Philander pebas , with partial separation of Brazilian sequences on the one hand from Peruvian and Ecuadorean sequences on the other ( fig. 16 View FIG ), but neither haplogroup received consistently strong support in our analyses. The only phenotypic evidence of geographic variation we observed was the caudal pigmentation of the easternmost specimens (from central Amazonia), most of which have ⅓ to ½ white tails, whereas those from western Amazonia usually have tails that are ≤¼ white.
COMPARISONS: Philander pebas is the only species in the genus with distinctly crenulated (folded and grooved) molar enamel, a trait that is most clearly visible on unworn teeth, but which persists on the lingual surfaces of the protocones even in old adults. Additionally distinctive traits, apparently unique among didelphids, are narrow enamel shelves along the anterolingual and posterolingual bases of the protocones; we refer to these shelves as the precingulum and postcingulum, respectively. 12 These shelves tend to wear away with age, but they often persist on M4 even in old adults. Another distinctive trait, only rarely observed as a polymorphism among other didelphids, is a narrow shelf along the posterolabial surface of the hypoconid; following standard tribosphenic terminology, this shelf is called the posterior cingulid or postcingulid.
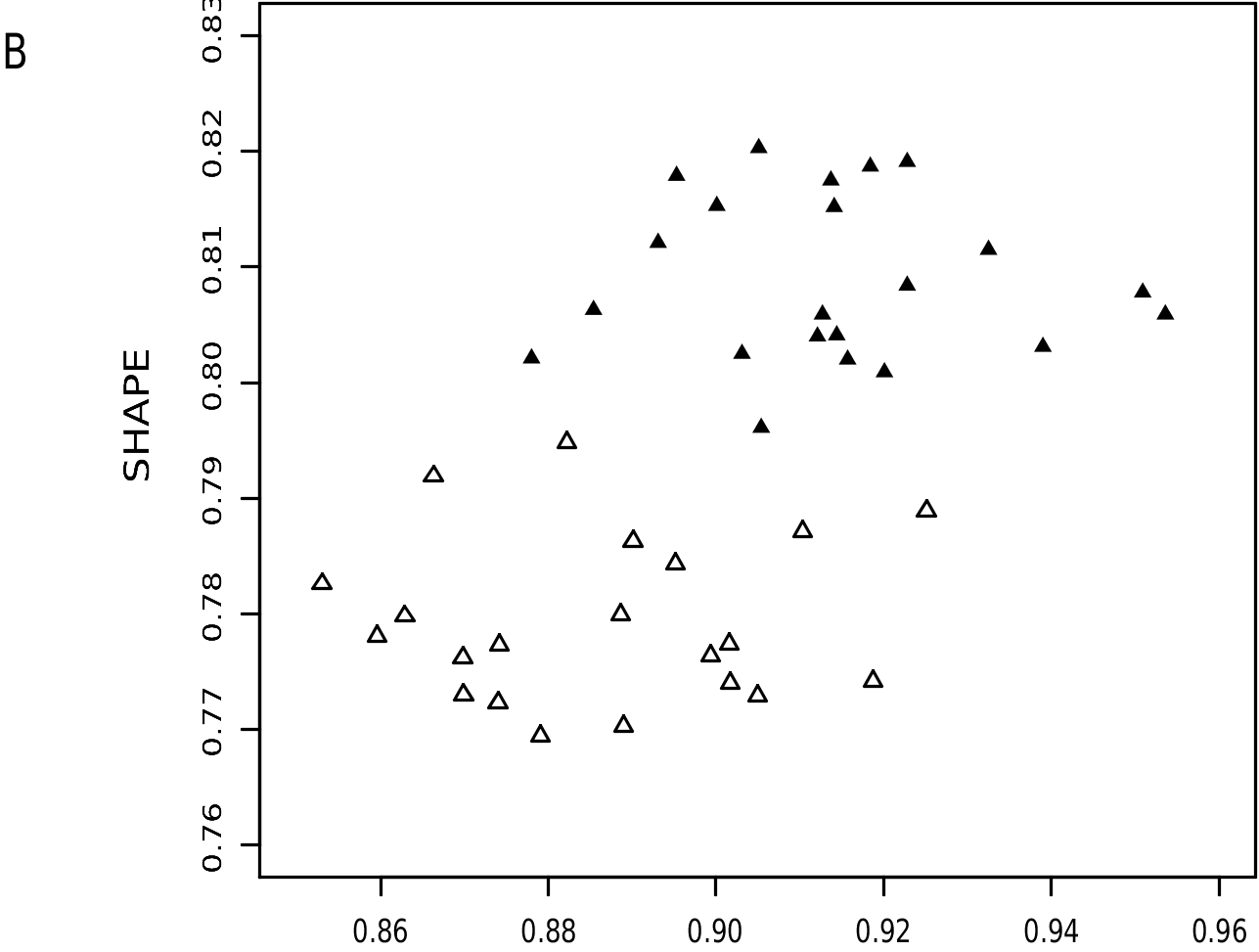
Philander pebas can be distinguished from its sister species, P. canus , by additional characters. Among others, it is substantially larger than P.canus ( tables 7, 8), and specimen scores on the first two principal components that we computed from craniodental measurements of both taxa illustrate nonoverlapping multivariate distributions ( fig. 21A View FIG ). Coefficients of general-size-invariant shape differences computed from these data suggest that P. pebas has longer but narrower nasals, wider interorbital and postorbital dimensions, and longer palates than P. canus ( fig. 21B View FIG , table 13). The two species can also be reliably identified by external traits, of which ventral pelage coloration is the most consistently useful. Whereas the ventral fur of P. canus is continuously self-whitish, -cream, or -buffy from chin to groin, the ventral fur of P. pebas is extensively gray-based. Some specimens of P. pebas have self-whitish or -buffy fur on the chin, throat, and/or groin, but none of the specimens we examined has a continuous midventral streak of self-colored fur over the chest and upper abdomen. The two species also seem to be reliably identifiable by tail markings in Ecuador, Peru, and Acre ( Brazil), where specimens of P. canus have tails that are at least ⅓ to almost ½ white, but where specimens of P. pebas have tails that are ≤¼ white.
Close comparisons of Philander pebas and P. quica seem unnecessary given their widely disjunct geographic distributions ( fig. 9 View FIG ), large genetic and morphometric distances (appendices 3, 4), and salient qualitative differences ( table 6).
Philander pebas differs from members of the P. opossum complex, with which it is broadly sympatric ( P. andersoni , P. mcilhennyi ) or potentially sympatric ( P. opossum ), by the unique dental traits described above and by external morphology. By comparison with P. andersoni —
12 There appears to be no standard terminology for these structures, despite their essential similarity among the tribosphenic taxa that exhibit them. Simpson (1936: 5), for example, used “anterior cingulum” and “posterior cingulum,” and the postcingulum is sometimes called the “talon” ( Bown and Kraus, 1979: 173).
with its distinctly blackish middorsal stripe ( fig. 10 View FIG )—the dorsal fur of P. pebas is uniformly grayish, although it can be indistinctly darker (sometimes almost blackish) middorsally. Additionally, where the ranges of P. andersoni and P. pebas overlap, they can readily be distinguished by tail markings (the scaly part of the tail of P. andersoni is ≥ ½ white, whereas the tail of sympatric P. pebas is ≤¼ white). By comparison with P. mcilhennyi (which is sometimes almost entirely blackish), P. pebas is uniformly grayish, and these species also differ in fur length: although observed ranges narrowly overlap, the middorsal fur of P. mcilhennyi is much longer on average (16 ± 3 mm) than the middorsal fur of P. pebas (10 ± 2 mm), and the latter species never has the typically shaggy appearance of P. mcilhennyi . As in P. andersoni , the scaly portion of the tail is at least ½ white in P. mcilhennyi , whereas the tail is mostly black in P. pebas . By comparison with P. opossum (which has mostly self-buffy underparts), the ventral fur of P. pebas is extensively gray-based (and is never buffy in the specimens we examined).
REMARKS: Specimens that we refer to Philander pebas were among those previously identified as P. opossum quica by Hershkovitz (1997), as P. opossum canus by Patton et al. (2000), as P. opossum by Woodman et al. (1991) and Hice and Velazco (2012), and as P. canus by Nunes et al. (2006). Although we were unable to examine any of the specimens from northeastern Peru identified as P. opossum by Díaz (2014), we suspect that most of them are P. pebas .
We have not examined specimens of Philander deltae (known only from northeastern Venezuela), but Lew et al.’s (2006) description of that species includes several external traits (including brownish dorsal fur, a broad strip of “uniformly cream” ventral fur, very small and poorly defined supraorbital spots, and sparsely pigmented ears) that are quite unlike the corresponding attributes of P. pebas . Because Lew et al. (2006) did not publish measurement data for P. deltae , no morphometric comparisons with P. pebas are possible.
HABITATS: All examined specimens of Philander pebas are from western and central Amazonian landscapes where the natural climax vegetation is lowland rainforest, but many localities in this region support a wide range of habitats. The floodplains of white-water rivers, in par-
PC1
SIZE ticular, typically include a mosaic of successional stages and edaphic formations ( Salo et al., 1986; Puhakka and Kalliola, 1995), and they are interdigitated with floristically distinct upland forests that grow on well-drained terraces and hillsides. Although fragmentary and incomplete, available ecological information from several localities suggest that P. pebas occupies a distinctive suite of natural and anthropogenic habitats within this diverse ecological matrix.
According to Patton et al. (2000), who trapped in both upland (terra firme) forest and seasonally flooded (várzea) forest along the Rio Juruá, Philander “ opossum ” was taken only in flooded forest, except in the headwaters region, where one specimen was trapped in upland forest. Of the 15 specimens of P. “ opossum ” they collected, we were able to examine only five, of which four were P. pebas and one was P.canus . All four specimens of P. pebas were taken at localities where the trapping habitat was described as várzea, by contrast with specimens of sympatric P. mcilhennyi , which the authors trapped in both terra firme and várzea habitats.
Another record of Philander pebas from seasonally flooded forest is based on the specimens of Philander “ canus ” analyzed by Nunes et al. (2006). These specimens, which we reidentified as P. pebas , were collected in the Mamirauá Sustainable Development Reserve, a protected area consisting entirely of várzea at the confluence of the Rio Japurá and the upper Amazon (Solimões). When the floodwaters are at their highest, virtually the entire reserve is flooded and only the forest canopy is visible above the water line ( de Queiroz and Peralta, 2010). During the low-water season, emergent land is covered by tall forest growing on levees, shrubby vegetation in lower areas, and a variety of other floodplain habitats ( Ayres, 1995). The specimens in question were trapped in seasonally flooded forest (C. Nunes, personal commun., 17 October 2017).
In addition to seasonally flooded riparian formations, this species has also been trapped in swamps (habitats with permanently waterlogged soils). Several specimens of Philander “ opossum ” have been collected in the vicinity of Cusco Amazónico, an ecotourist lodge on the Río Madre de Dios in southeastern Peru ( Woodman et al., 1991). Although Cusco Amazónico is located within the meander belt of the Madre de Dios, the various habitats sampled by zoological collectors at this locality were not seasonally flooded by river water ( Duellman, 2005). Of the two specimens of P. pebas that we examined from Cusco Amazónico—where P.canus also occurs— only one is accompanied by definite habitat information. This specimen (KU 1441209) was collected in a Heliconia swamp; judging from information provided by Duellman (2005), the capture site is probably seasonally inundated by accumulated rainwater in the wet season.
Lastly, this species has been collected in anthropogenic habitats on well-drained soils. According to Hice and Velazco (2012), who reported on material collected in the Reserva Nacional Allpahuayo-Mishana and at the nearby Fuerte Militar Otorongo in northeastern Peru, Philander “ opossum ” was collected only in secondary vegetation and agricultural fields, whereas P. andersoni occupied adjacent primary forest habitats. We examined 16 of the 39 specimens of P. “ opossum ” reported by these authors, and all were examples of P. pebas .
Based on these scant data, we hypothesize that Philander pebas is primarily a várzea species; that is, one that typically inhabits riparian formations seasonally flooded by white-water rivers (for Amazonian flooded-forest nomenclature, see Prance, 1979). In support of this conjecture, we note that the geographic distribution of the species ( fig. 9 View FIG ) corresponds closely to the distribution of white-water catchments in the Amazon Basin ( Junk et al., 2011: fig. 1 View FIG ), and we boldly predict that P. pebas will eventually be found to inhabit the white-water Caquetá and Putumayo drainages of southeastern Colombia, from which we have yet to examine any material. Because várzea habitats are characterized by riverine flooding, terrestrial (nonaquatic and nonarboreal) species that inhabit such forests during the low-water season must periodically migrate to higher ground, and the ability to occupy temporary refugia may preadapt terrestrial várzea species to also utilize swampy habitats (seasonally flooded by accumulated rainwater), as well as to opportunistically invade secondary vegetation resulting from human activity on adjacent terraces and hillsides.
ETYMOLOGY: After Lago Pebas, the vast Miocene lake complex ( Wesselingh et al., 2001) or “mega-wetland” ( Hoorn et al., 2010) that filled much of the Andean foreland basin, including almost the entire known geographic range of this morphologically distinctive species.
SPECIMENS EXAMINED (N = 58): Brazil — Acre, Fazenda Santa Fé (on Rio Juruá; MVZ 190345), opposite Ocidente (on Rio Juruá; MVZ 190346); Amazonas, Igarapé Nova Empresa (on Rio Juruá; MVZ 190343), Lago do Baptista (on S bank of Amazon; FMNH 51095), Sacado (on Rio Juruá; MVZ 190344), Santo Isidoro [near] Tefé (on S bank of Amazon; AMNH 78954), Parintins (“Villa Bella Imperatriz,” on S bank of Amazon; AMNH 92880, 92881, 93526–93528, 93968), Tapauá (on Rio Purus; USNM 461374). Ecuador — Orellana, 42 km S Pompeya Sur (ROM 106101, 106139). Peru — Loreto, Apayacu (AMNH 74388), Avícola San Miguel (MUSM 33590, 33592, 33593), Cabo López (MUSM 33566, 33567, 33569, 33570, 33572), Carretera Iquitos-Nauta km 28.8 (MUSM 34892), Caserio Cahuide (MUSM 33564, 33574, 33576), El Paujil (MUSM 33580), El Triunfo (MUSM 33586, 33587, 33583), Iquitos (AMNH 98642), 19.7 km SW Iquitos (MUSM 33588), Mishana (MUSM 33597), Orosa (AMNH 73852), Otorongo Army Base (LACM 91621, 91622), Peña Negra (MUSM 33598), Picuro Yacu (MUSM 33594), Quistococha (FMNH 122745–122748; MUSM 33599, 33600), San Gerardo (MUSM 33602), Santo Tomas (MUSM 33603), Sarayacu (on Río Ucayali; AMNH 76448–76450); Madre de Dios, Cusco Amazónico (KU 144120, 144121; MUSM 6074); Ucayali, Balta (LSUMZ 12007, 12010, 14011), Yarinacocha (FMNH 55411).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |