Deplorothrips Mound & Walker
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4208.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:8F4AF129-0A68-4EBC-AF85-06F634EC3897 |
|
DOI |
https://doi.org/10.5281/zenodo.6078210 |
|
persistent identifier |
https://treatment.plazi.org/id/3745563A-4F3F-FFAA-37C9-FEC7D4B0FAA5 |
|
treatment provided by |
Plazi |
|
scientific name |
Deplorothrips Mound & Walker |
| status |
|
Deplorothrips Mound & Walker View in CoL
Deplorothrips Mound & Walker, 1986: 49 View in CoL . Type species D. bassus Mound & Walker View in CoL
The pattern of character states exhibited by the new species described below is interpreted as indicating that these species represent a single lineage that has radiated within Australia. These species, with two exceptions, conform satisfactorily to the original generic diagnosis of Deplorothrips View in CoL , as well as the diagnosis published by Okajima (2006). However, there is remarkable variation in many character states between some of the species. Rather than produce another long and confusing generic diagnosis noting the many differences in particular character states, variation in each of the major characters is here discussed individually. In considering this variation, it must be stressed that there is often a lack of correlation between the various states, such as maxillary stylet retraction and form of antennal segment VIII. This lineage of Australian and eastern Asian species that is designated the genus Deplorothrips View in CoL is presumably derived from within the “ Phlaeothrips View in CoL -lineage” (sensu Mound & Marullo 1996) and is most closely related to the genus Hoplothrips View in CoL . The maxillary stylets in Deplorothrips View in CoL species are wide apart in the head, and in most species are less retracted into the head capsule than most Phlaeothripinae View in CoL . Species of Hoplothrips View in CoL and related genera including Acanthothrips View in CoL , Hoplandrothrips View in CoL and Phlaeothrips View in CoL , all have the stylets deeply retracted, at least to the level of the postocular setae, and close together medially almost for the full length of the head (see figures in Mound & Walker 1986; Mound & Tree 2013). One exception to this generalisation is the minute North American species, Hoplothrips smithi View in CoL , in which the stylets are short and low in the head, but in the absence of any modern account of the New World species of fungus-feeding Phlaeothripinae View in CoL it is not possible to comment on the relationships of this species.
Character state variation. Body colour: Most species are medium to light brown with red internal pigments. The hind tibiae are clear yellow in many species, but light brown, or at least washed with brown, in some of the most common species in Australia. The antennal segments are generally brown with III paler in most species, although segment III is clear yellow in a few species, and most of the specimens in the bassus -complex from New Zealand have antennal segment III brown. The major body setae are consistently pale on all of the species considered here, with the exception of retis .
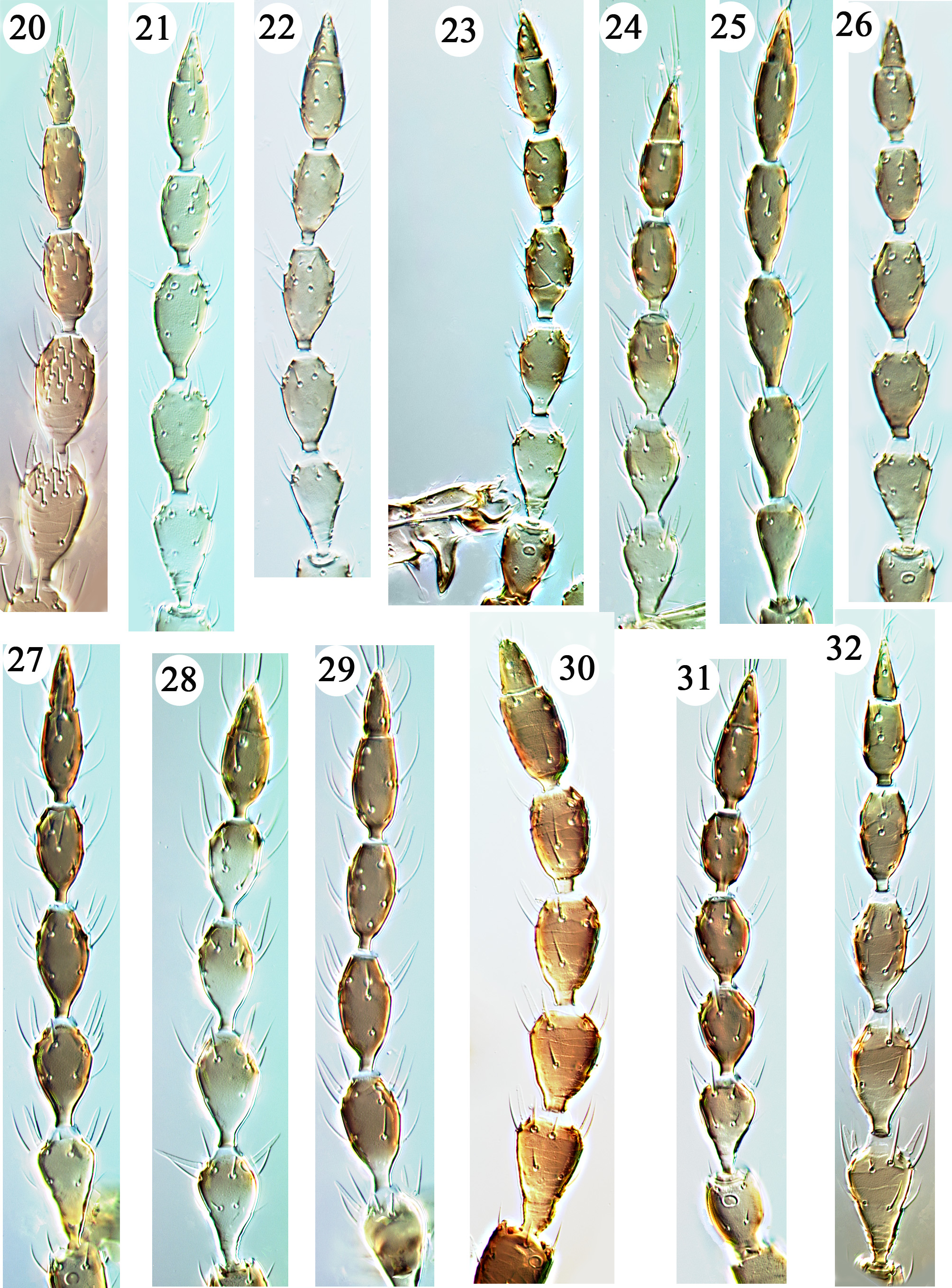
Antennal segmentation: Eight segments are present in all species, with segment VIII usually broadly based (almost fused to VII in two species from southern Japan), but narrowed to the base in two species described below, and in one of these almost lanceolate ( Figs 20–32 View FIGURES 20 – 32 ).
Antennal sense cones: Segment III bears either 1, 2 or 3 sense cones (= sensilla basiconica), whereas IV bears 2, 3 or 4. In some species the macropterae bear more and larger sense cones than micropterae or apterae, and in one of these species macropterae have numerous small sensory hairs (= sensilla trichodea) ventrally on segments IV–VI ( Fig. 20 View FIGURES 20 – 32 ). A remarkable variant is the presence on some specimens at the inner apex of segment IV of either two small sense cones or of one large sense cone, and bilateral asymmetry in this occurs in some individuals. Sense cones occasionally fall off during slide preparation, and it is essential to look for the basal pores of these structures to determine their presence or absence. Variation in the number of sense cones on antennal segment IV is also known to occur in the widespread predator, Karnyothrips flavipes (see Okajima 2006).
Head cheek setae: The typical condition involves one or two pairs of rather weak cheek setae ( Figs 1–7 View FIGURES 1 – 7 ), but major males can have several pairs with some setae considerably stouter ( Fig. 17 View FIGURES 14 – 19 ). In a few species the cheek setae are weak and scarcely apparent.
Mouth cone length: The mouth cone is usually short and rounded, but in a few species it is pointed and longer, extending beyond the fore coxae ( Figs 18–19 View FIGURES 14 – 19 ).
Maxillary stylet position: Although low in the head in typical Deplorothrips species ( Okajima 2006), there is considerable variation. The images of heads ( Figs 1–13 View FIGURES 1 – 7 View FIGURES 8 – 13 ) are here presented in the order of increasing stylet retraction. This emphasises that there is a progression from stylets not retracted anterior to the occipital ridge, to stylets retracted to the level of the postocular setae, with no clear distinction between the various conditions. The maxillary levers, articulating at the anterior end of the straight and rigid maxillary pillars, are commonly orientated in Phlaeothripinae along the longitudinal axis of the head ( Fig. 9 View FIGURES 8 – 13 in Heming 1993). Among Deplorothrips species, this condition is found only in retis sp.n., a species that, as discussed below, is only weakly related to the other members of the genus. In most species of Deplorothrips the levers are orientated at an angle toward the mid-line ( Fig. 13 View FIGURES 8 – 13 ), and in many species they are also not in a horizontal plane but are directed dorsally and thus appear foreshortened ( Figs 1–7 View FIGURES 1 – 7 ). Slide-mounting procedures often result in the stylets becoming disrupted, and in such specimens it may not be possible to predict the natural retracted condition. When seriously disrupted, the levers may be orientated almost transversely or even directed posteriorly, and the stylets are not straight but slightly wavy.
Fore tarsal tooth: This is present in both sexes, although varying greatly in size and shape. In some individuals it is short and sharply pointed, but in large males it is bluntly pointed and at least as long as the fore tarsal width.
Male fore tibia: The inner apex bears a small tubercle in males of most species, but this tubercle is larger in major males than in small males ( Figs 23 View FIGURES 20 – 32 , 45 View FIGURES 33 – 47 ).
Pronotum: Although transverse in females, the pronotum of large males is more elongate with a stout longitudinal median apodeme ( Figs 14–16 View FIGURES 14 – 19 ). The notopleural sutures are almost always complete.
Pronotal setae: Four pairs of major setae are always developed and capitate, but the am setae are usually acute and no larger than discal setae in both sexes. However, in females of a few species the am setae are capitate although rarely more than half as long as the aa setae ( Figs 1, 4 View FIGURES 1 – 7 ).
Thoracic sternites: Prosternal basantra are not present in any species, and the ferna are transverse and often almost meeting in the midline ( Figs 18–19 View FIGURES 14 – 19 ). The mesopraesternum is sometimes transverse in macropterae, but is eroded in most individuals to a pair of small lateral sclerites. Metathoracic sterno-pleural sutures are present in all individuals.
Metanotum: In most species of the genus, whether macropterous or apterous, the metanotum lacks sculpture, although in two species it is reticulate ( Figs 1 View FIGURES 1 – 7 , 36 View FIGURES 33 – 47 ), and in a few species the posterior area of the metanotum of large males bears curious longitudinal sculpture ( Figs 5 View FIGURES 1 – 7 , 33, 37 View FIGURES 33 – 47 ). In one species, the metanotum bears many small setae.
Fore wing condition: Macropterae are generally uncommon in this genus, but when present the fore wings are almost parallel sided, and bear no more than 12 duplicated cilia. Apterae usually have no indication of a wing lobe, but apterae in a few species have a minute round lobe about 5 microns in diameter, and micropterae of some species have the wing lobe less than 25 microns long. These small lobes sometimes bear one or two setae. There is thus no simple distinction between winged and wingless conditions. Moreover, although micropterae and apterae usually lack ocelli, some such specimens have paired ocelli, or even just one, weakly developed posterior ocellus.
Pelta: The shape of the first abdominal tergite is rather indeterminate in the species considered here. In a few macropterae it approaches a typical Hoplandrothrips condition ( Mound & Tree 2013), but in most individuals it is shorter and broader, ranging from sub-quadrate to D-shaped, and often with the anterolateral margins partially eroded ( Figs 9–10 View FIGURES 8 – 13 ; 14–16).
Tergite IX setae: Setal pairs S1 and S2 are capitate and shorter than the tube in most species, sometimes no longer than the basal width of the tube. However, in one species they are pointed and longer than the tube. The accessory setal pair between S1 and S2 is usually long ( Dang et al. 2013).
Tergal wing-retaining setae: The typical two pairs of sigmoid setae are present in all species studied, but although they are large and sigmoid in macropterae, they are short, straight and acute in micropterae and apterae.
Male sternites III–VI: The largest males of some species usually have transverse rows of specialised reticulation anterolaterally on these sternites ( Figs 42, 46, 47 View FIGURES 33 – 47 ); these are presumably associated with glandular tissues ( Okajima 2006). Small males of the same species usually lack these structures.
Male sternite VIII: Typically the males in this genus have a small pore plate, but this varies in shape between species from small and oval to slender and transverse, although in one species it is broadly transverse ( Figs 38–44 View FIGURES 33 – 47 ), and males of one species lack any pore plate.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Phlaeothripinae |
Deplorothrips Mound & Walker
| Mound, Laurence A. & Tree, Desley J. 2016 |
Deplorothrips
| Mound 1986: 49 |