Plagiometriona forcipata ( Boheman , 1855)
|
publication ID |
https://doi.org/10.1080/00222930903528230 |
|
persistent identifier |
https://treatment.plazi.org/id/3948217D-402A-FF86-FE4B-146CFDFAFAC7 |
|
treatment provided by |
Carolina |
|
scientific name |
Plagiometriona forcipata ( Boheman , 1855) |
| status |
|
Plagiometriona forcipata ( Boheman, 1855) View in CoL
Coptocycla forcipata Boheman, 1855: 198 ( type locality: ‘Brasilia’).
Coptocycla emarcida Boheman, 1855: 342 ( type locality: ‘Brasilia’), syn. nov.
Life cycle and seasonal variation in adult field populations
Observations in the field recorded a total of 26 matings, 16 occurring among P. emarcida individuals, two among P. forcipata individuals and eight among individuals of both species ( Fig. 1A View Figure 1 ). Because individuals could be sexed with confidence during matings, we were able to observe that both species occurred within each sex on the same host plant species and at the same time of year.
Eggs were deposited either singly or in small groups (maximum of four), mostly on the undersides of leaves and were covered by a thin transparent membrane typical of most Plagiometriona species ( Fig. 1B View Figure 1 ). Unidentified species of eulophid wasps were occasionally observed standing on the elytra of females, and on one occasion, on the elytra of an ovipositing female ( Fig. 1C View Figure 1 ). Larvae constructed a fecal–exuvial shield, which they carried above their dorsum as in other genera of Cassidinae ( Fig. 1D View Figure 1 ) and which was retained during pupation ( Fig. 1E View Figure 1 ). Teneral P. emarcida adults were greenish ( Fig. 1F View Figure 1 ), whereas P. forcipata individuals emerged with a visible annulus, which darkened over time, first on the pronotum and then on the elytra. Adults of both species in the field were found mainly on the underside of host plant leaves (82.2% and 82.9% for P. emarcida and P. forcipata individuals, respectively; n = 213 and n = 41).
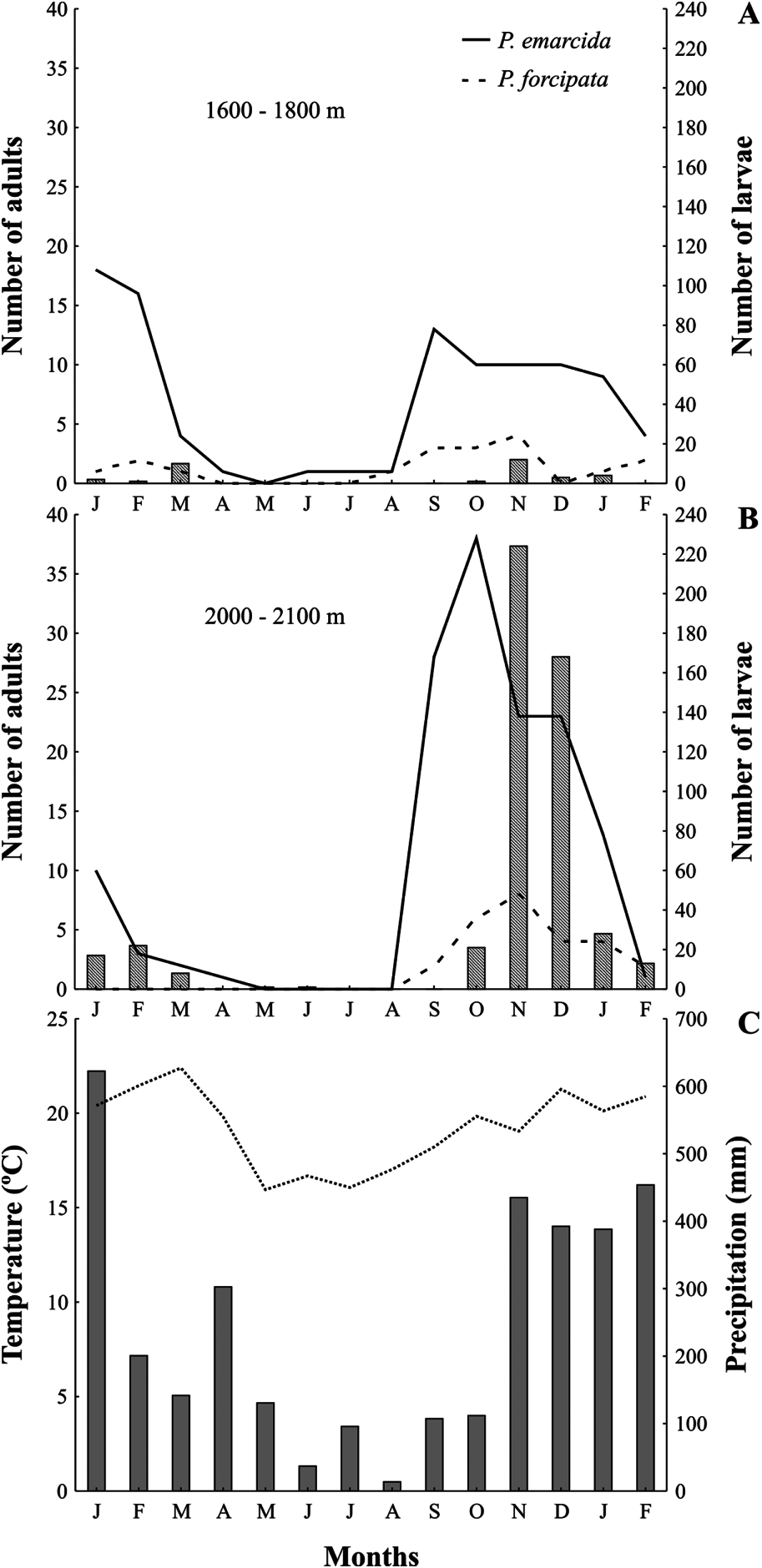
Finally, surveys in the study areas revealed that adult abundance of the two species varied in a similar manner throughout the year and at both altitudinal sites. Adult individuals of P. emarcida were always more common than P. forcipata individuals (240 versus 44, respectively). Adults of both species were more numerous from September to December, showed a decrease in abundance at the beginning of the year and were rare from April to August in both altitudinal sites ( Fig. 2A,B View Figure 2 ). This pattern follows that described for other Chrysomelidae species in the area and at the same altitude ( Flinte et al. 2009). Larvae became abundant on food plants 1–2 months later than adults, but otherwise were distributed across the year similar to adults, being rare from April to September, numerous from October to February, and peaking in November ( Fig. 2A,B View Figure 2 ). Temperatures were lower from May to August and higher from December to March, while rainfall was scarce from June to August and elevated from November to February ( Fig. 2C View Figure 2 ). Temperature and rainfall therefore seem to correlate closely with the seasonal distribution of these species. The onset of harsher climatic conditions was correlated with decreasing abundance in both species, whereas the onset of milder conditions in spring and summer correlated with increased numbers, first of adults then of immatures.
Although observations of adults of both species were more frequent at the highelevation site ( 142 P. emarcida and 26 P. forcipata versus 98 and 18 at mid-elevation, respectively), the overall representation of the species was identical at the two sites, 84.5% P. emarcida and 15.5% P. forcipata individuals. Individuals of both species were more widely distributed across the year at the mid-altitude site (13 and 9 months for P. emarcida and P. forcipata , respectively, compared with 10 and 6 months at high elevation) ( Fig. 2A,B View Figure 2 ). Moreover, the greater abundance of larvae and adults at the high-altitude site occurred during the warmer months of the year. Also, although adults of both species were rare during the less favourable season, they disappeared entirely from censuses only at the high-altitude site ( Fig. 2A,B View Figure 2 ), where temperatures were considerably lower than at the mid-elevation.
Development and mortality of offspring reared in the laboratory from the ovipositions of field-collected females
Plagiometriona emarcida females deposited 4.6 ± 0.9 eggs/day ( n = 7) and P. forcipata females deposited 5.7 ± 1.7 eggs/day ( n = 8), rates which were not significantly different ( t = − 1.53; 13 d.f.; p = 0.14). The lengths of the egg and larval stages were significantly greater in offspring of P. forcipata females, resulting in a significant 1.8-day difference in the length of immature development between the species ( Table 1). Larvae hatched 10–11 days after oviposition and the overall life cycle (oviposition to adult emergence) in captivity lasted ca. 41.3 days for offspring of P. emarcida females and approximately 43.1 days for offspring of P. forcipata females. Sclerotization of teneral adults ( Fig. 2F View Figure 2 ) of both species took 13.4 ± 2.8 days (n = 14).
Mortality in the laboratory during each developmental period was significantly higher for offspring of P. forcipata females; 14.5% versus 24.8% during the egg stage (chi-square = 4.9; 1 d.f.; p <0.027), 14.5% versus 41.2% during the larval stage (chi-square = 18.4; 1 d.f.; p <0.000) and 2.5% versus 38.6% during the pupal stage (chi-square = 58.6; 1 d.f.; p <0.0001) for P. emarcida and P. forcipata individuals, respectively ( Table 2). The total mortality rate from egg to adult was also significantly different, 29.2% versus 73.7% for P. emarcida and P. forcipata individuals, respectively (chi-square = 28.83; 1 d.f.; p <0.001).
In neither species did the adult sex ratio of the F1 generation vary significantly from an expected 1: 1 ratio ( P. emarcida offspring: 77 males and 95 females, chi-square = 1.88, 1 d.f., p <0.170; P. forcipata offspring: 32 males and 23 females, chi-square = 1.47, 1 d.f., p <0.225). Nor did the sex ratio differ significantly between the two species (chi-square = 3, 1 d.f., p <0.0831) and when combined, the overall numbers of males (109) and females (118) were remarkably similar (1: 1.08).
Frequencies of the species in adult field populations and in offspring reared
in the laboratory
Plagiometriona emarcida individuals constituted 84.5% of the 284 individuals sighted on the field censuses. The 10 P. emarcida and three P. forcipata field-caught females moved to the laboratory produced a total of 353 offspring, 80.2% of which were P. emarcida individuals, proportions not significantly different from those observed in the field (chi-square = 2.01, 1 d.f., p = 0.16). The colour pattern of offspring from both “species” overwhelmingly resembled that of their mothers but a minority of individuals were of the alternative colour pattern ( Table 3).
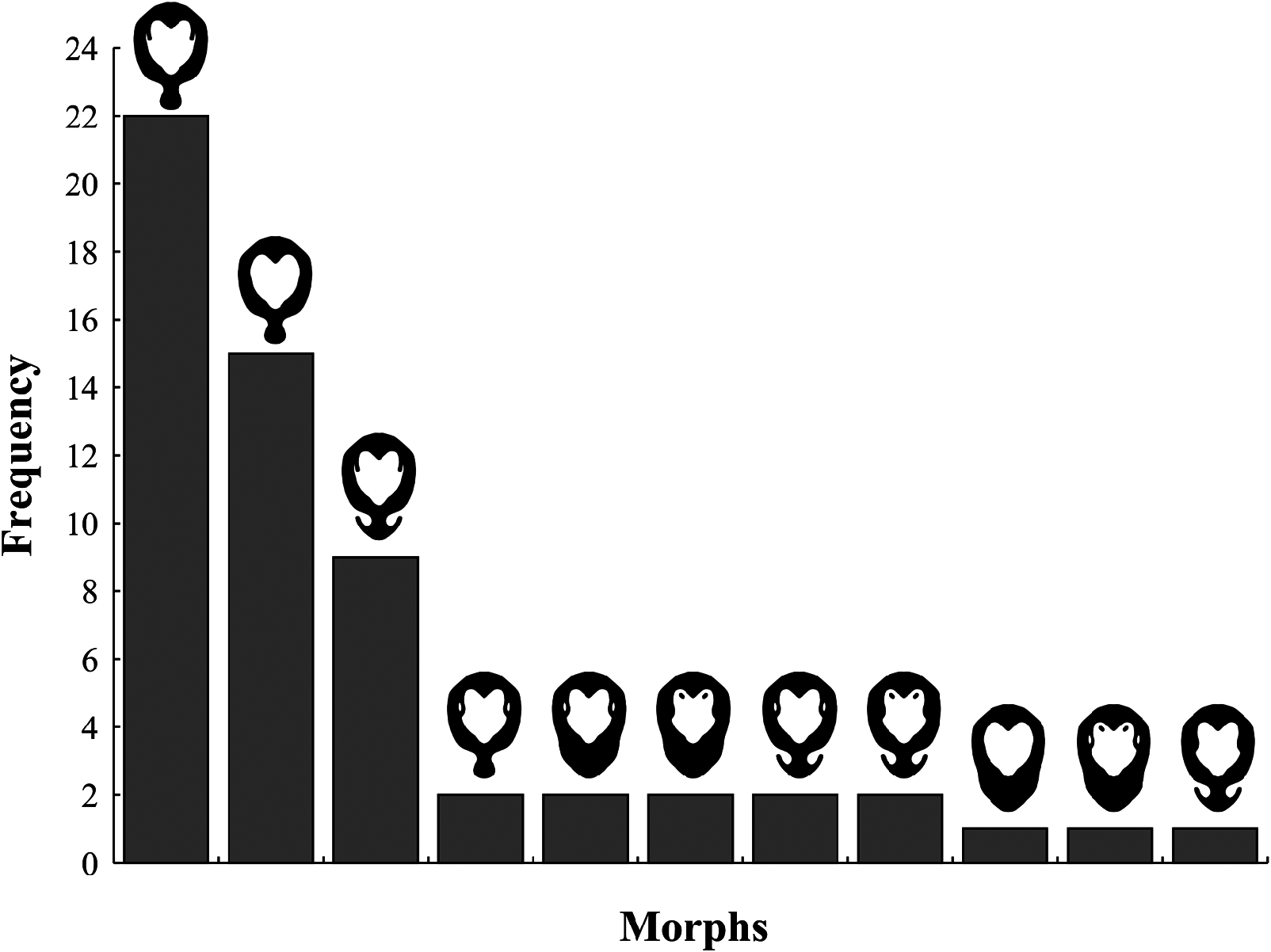
Phenotypic variation within P. forcipata
At least 11 variants in the expression of the dark annulus on the elytral disk and pronotum were found among P. forcipata offspring ( Fig. 3 View Figure 3 ). The two most frequent variants recorded in the F1 offspring were also the two most common variants casually observed during field observations.
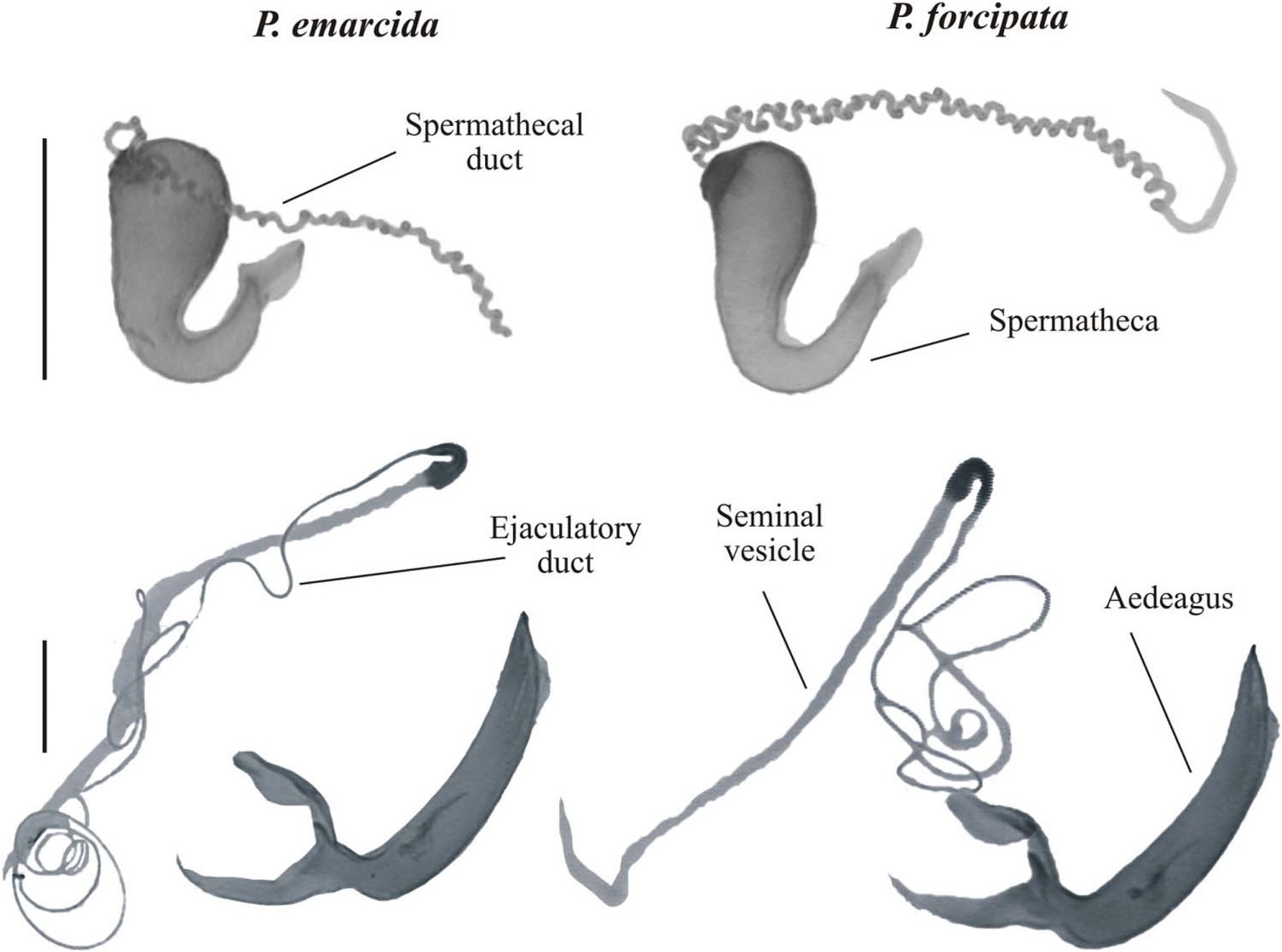
Genitalia
There were no obvious between-species differences in the shape or size of male or female genitalia ( Fig. 4 View Figure 4 ). The spermatheca was well sclerotized and falcate and the spermathecal duct was long (approximately 10.5 mm) and loosely coiled along almost its entire length in each species. The uncoiled male ejaculatory duct was approximately 7 mm in length, the seminal vesicle was 2.6 mm in length and the aedeagus was greenish in colour in fresh specimens and 2.0 mm in length (straight line distance, base to tip) and 0.3 mm at maximum width.
Sequencing
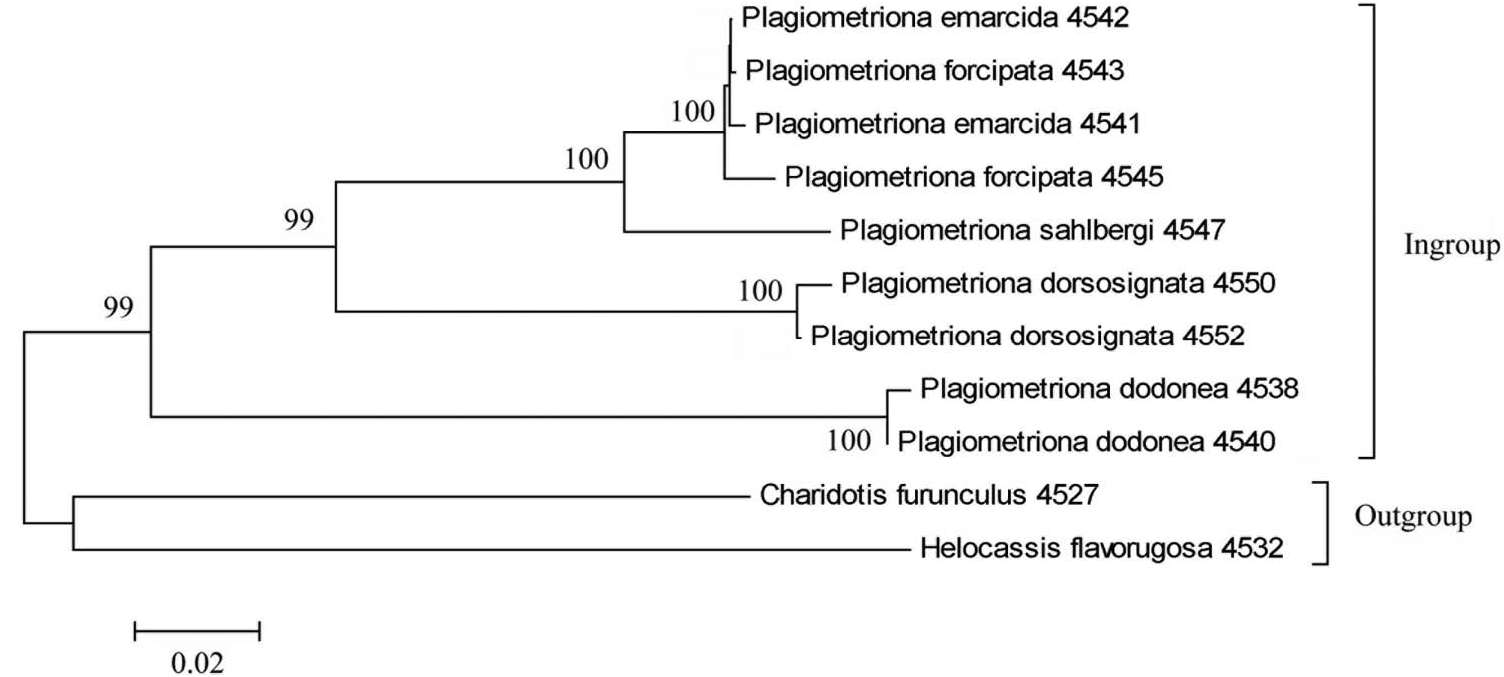
We used 1179 base pairs of sequence data obtained from the mitochondrial cytochrome oxidase gene ( CO1) to examine the divergence between P. emarcida and P. forcipata relative to a small number of congeners and two outgroup taxa within the tribe Cassidini . Nucleotide sequences were identical for both P. forcipata samples except for an ambiguous read occurring at positions 39–42 in P. forcipata no. 2. Accordingly, a single amino acid gap was generated at position 14, the only difference existing in the two P. forcipata protein sequences. Nucleotide sequences for both P. emarcida individuals were identical, and differed from the P. forcipata sequences only at position 513, where an A was present in both P. emarcida and a C was present in both P. forcipata sequences. This third position mutation had no effect on the amino acid (threonine) produced by this codon in either morph. A great deal more collecting and sequencing would be required to determine whether this mutation is a fixed genetic difference occurring between the two morphs. The neighbour-joining tree ( Fig. 5 View Figure 5 ) shows no significance divergence between P. emarcida and P. forcipata , clear indication that these taxa belong to the same biological species. In contrast, Plagiometriona sahlbergi , the next closest taxon within this small set of species, varies by 5–6%.
Taxonomic comments
Originally, both species were placed in the fourth group of Spaeth’s Plagiometriona system, characterized by humeral angles normally protruding anterad, epipleural margin not reaching to apical margin of elytra, elytra regularly convex, second and third antennomeres subequal in length and basal antennomeres uniformly yellow, pronotum more or less quadratic with more or less distinct anterior corners, and terminal antennomere black. Spaeth (1937) included in the group five species, three of them from Brazil, P. forcipata and P. emarcida and P. gyrata ( Boheman, 1855) . Plagiometriona gyrata differs in having a subcircular body and regularly rounded lateral sides of the elytra, while P. forcipata and P. emarcida have oval elytra with lateral sides distinctly emarginate in the anterior third. For separation of P. forcipata and P. emarcida there is no criterion other than the colour of elytra.
Type material examined
Coptocycla emarcida . Lectotype [designated by Borowiec (1999)], pinned: “Brasil [white, printed and soft label] // Bhn. [white, printed and soft label] // Type [white, printed and soft label] // LECTOTYPE / des. L. Borowiec [red, printed and cardboard label]”; paralectotype, pinned: “ Brasil [white, printed and soft label] // M. Gall [white, printed and soft label] // PARALECTOTYPE / des. L. Borowiec [red, printed and cardboard label]” (both specimens preserved at Naturhistoriska Rijksmuseet, Stockholm, Sweden) .
Coptocycla forcipata . Lectotype [designated by Borowiec (1999)], pinned: “Brasil [white, printed and soft label] // Germ. [white, printed and soft label] // Type [white, printed and soft label] // LECTOTYPE / des. L. Borowiec [red, printed and cardboard label]”; paralectotype, pinned: “ Brasil [white, printed and soft label] // Bhn. [white, printed and soft label] // PARALECTOTYPE / des. L. Borowiec [red, printed and cardboard label]”; paralectotype, pinned: “Brasil [white, printed and soft label] // Bhn. [white, printed and soft label]” (all specimens preserved at Naturhistoriska Rijksmuseet , Stockholm, Sweden).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Plagiometriona forcipata ( Boheman , 1855)
| Flinte, Vivian, Windsor, Donald, Sekerka, Lukas, Macedo, Margarete Valverde de & Monteiro, Ricardo Ferreira 2010 |
Coptocycla forcipata
| Boheman, CH 1855: 198 |
Coptocycla emarcida
| Boheman, CH 1855: 342 |