Microcaecilia taylori Nussbaum & Hoogmoed, 1979
|
publication ID |
https://doi.org/10.5281/zenodo.203509 |
|
DOI |
https://doi.org/10.5281/zenodo.5635417 |
|
persistent identifier |
https://treatment.plazi.org/id/3E0BA842-4D42-2A4B-BD96-D2581D6FF863 |
|
treatment provided by |
Plazi |
|
scientific name |
Microcaecilia taylori Nussbaum & Hoogmoed, 1979 |
| status |
|
Microcaecilia taylori Nussbaum & Hoogmoed, 1979
Microcaecilia sp. “A” Hoogmoed, 1979: 273.
Microcaecilia taylori Nussbaum & Hoogmoed, 1979: 245 ; Frost, 1985: 623; Reynolds et al, 2004; Frost, 2008. Microcaecilia sp. Caldwell & Araújo, 2005: 6.
Diagnosis (modified from Nussbaum & Hoogmoed, 1979): Maximum known TL 225 mm (MPEG 22157). PA 113–130. SG 0–21. Eye not visible. PPT mono- or bicuspid, even in the same specimen, the second condition predominating in specimens with both conditions; maxillary teeth extend posteriorly beyond level of choanae; a weak vertical terminal keel may be present. PPT may be absent in juveniles. A small terminal shield, at most one annular groove posterior to the vent. Dermal scales starting between annulus 34 and 121; absent in juveniles. Subdermal scales absent.
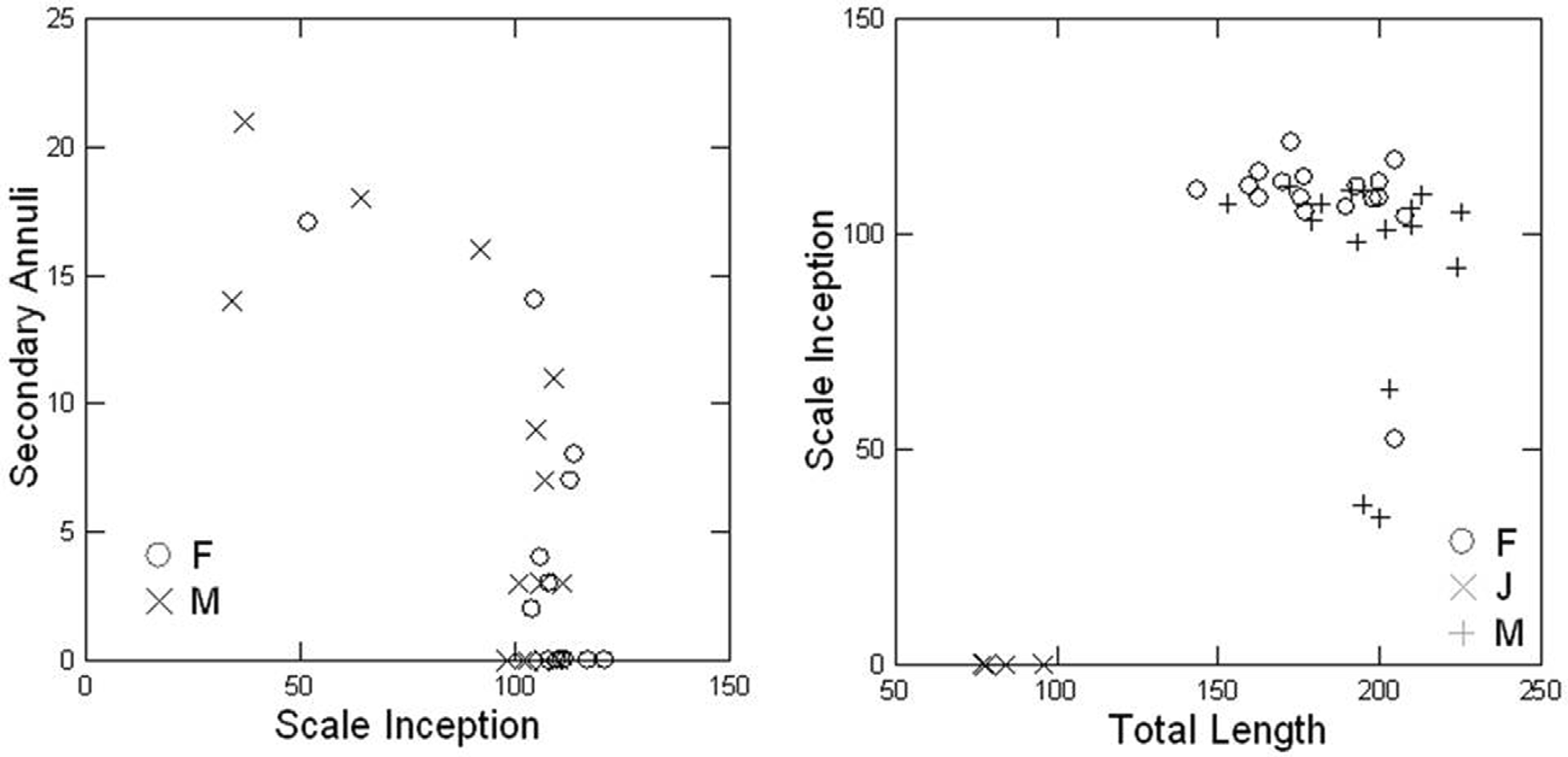
Description. TL 25.7 to 52.4 times (39.8 ± 4; n = 89) BW. Head slightly narrower than the body. Snout projecting distinctly beyond mouth. Eyes not visible, not even in smallest juveniles. Nuchal grooves may be distinct dorsally and ventrally, except third which ventrally is incomplete; second nuchal collar partially fused below with first primary fold; a dorsal transverse groove is present on each collar, shorter and less distinct on first. Body subcylindrical, slightly wider than deep. Width along body may vary slightly.
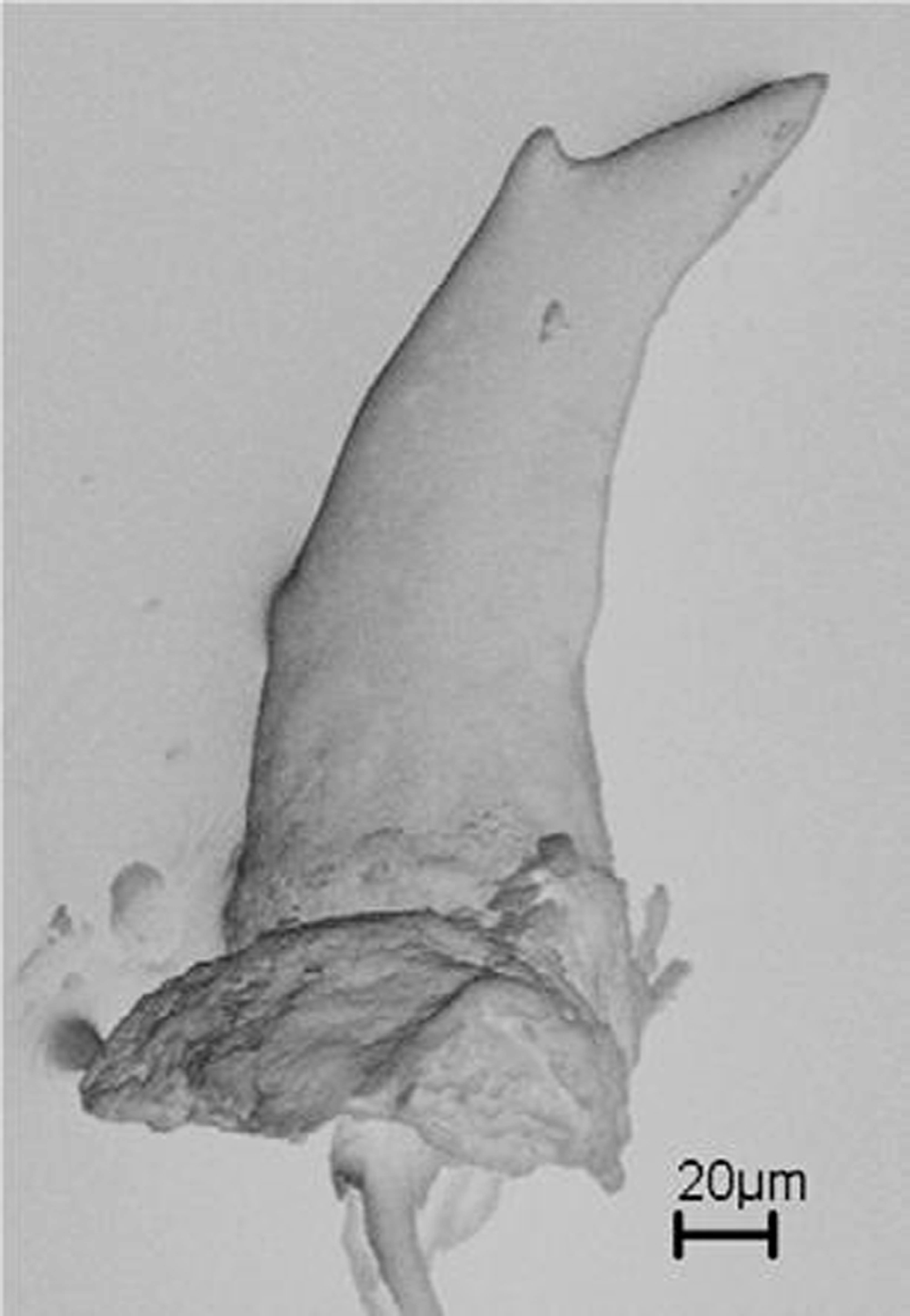
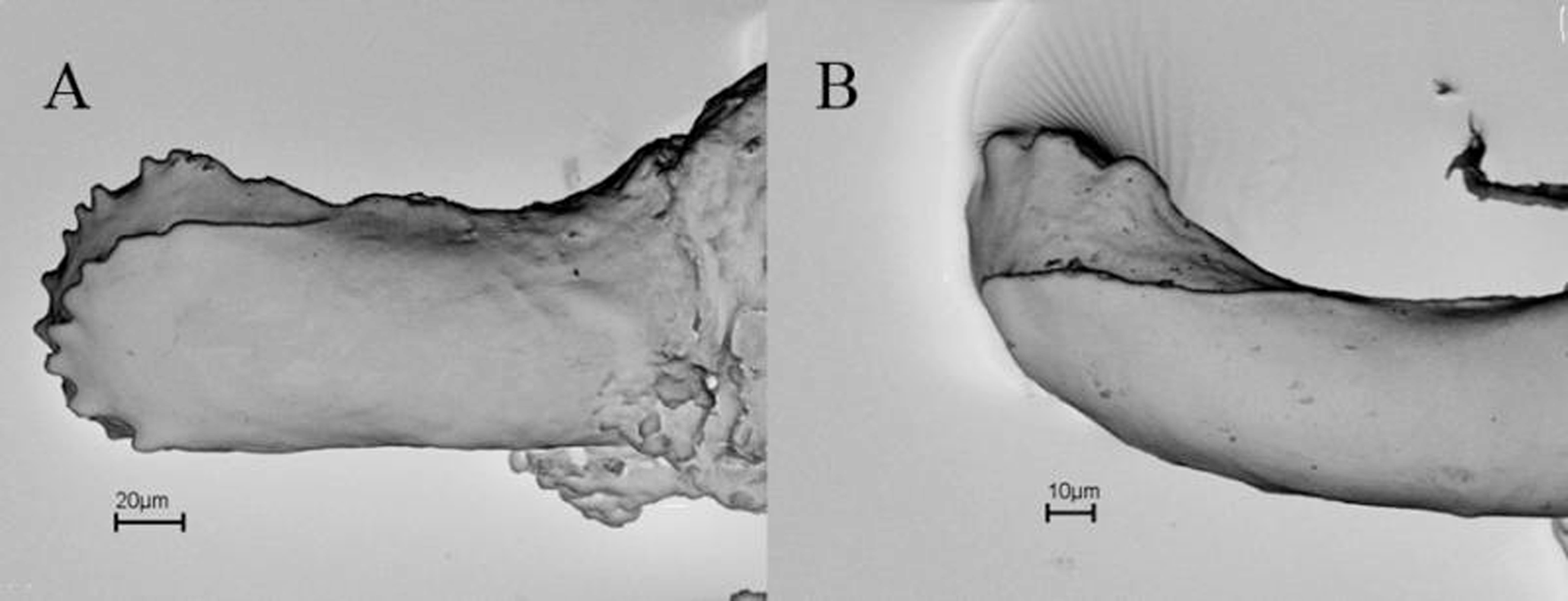
Primary annular grooves completely encircling body, except anteriormost primary groove that is ventrally incomplete, as well as some grooves at midbody and interrupted by vent. At most six secondary grooves complete. Paired anal papillae in two of 46 male specimens analyzed; absent in all 55 females analyzed for this character. Terminal shield on posteriormost part of body; generally no annular groove posterior to vent, but sometimes there is one. Weak vertical keel may be present on terminal shield. Vent transverse, sometimes slightly irregularly shaped. AD 9–16; generally about as many on anterior as on posterior edge of vent (e.g. six anterior, six posterior in MPEG 23365). Dermal scales starting between annulus 34 and 121; at most nine rows per fold. No marked variation of SI in relation to TL, but smallest specimens (e.g. MPEG 7352; 22171; 22172; 22783) did not have dermal scales, demonstrating ontogenetic variation ( Fig. 14 View FIGURE 14 ). No marked relation between number of SG and SI, but specimens with more SG have a low SI ( Fig. 14 View FIGURE 14 ). There is no apparent sexual difference in SI ( Fig. 14 View FIGURE 14 ). In small juveniles PPT may be absent. PMT monocuspid, maximally 30 ( Nussbaum & Hoogmoed, 1979) with little variation in size, but posterior maxillary teeth slightly smaller, extending to level of posterior border of the choanae or posteriorly of them. Maximally 30 PPT ( Nussbaum & Hoogmoed, 1979), which may be mono- and bicuspid in one specimen or only bicuspid ( Fig. 15 View FIGURE 15 ), teeth smaller than PMT, no apparent variation in size. DT monocuspid, maximally 23, approximately of same size as PMT. “Fetal” teeth in small juveniles (MPEG 22171 with TL 77 mm and MPEG 22172 with TL 78mm). MPEG 22171 has three distinct tooth series, typical of Microcaecilia ; counts did not differ much from adult counts, with 18 monocuspid PMT, 16 mono- or bicuspid PPT and 18 DT with two flattened cusps (fetal teeth). MPEG 22172 has only two tooth series; 16 mono- or bicuspid PMT and 12 DT with two flattened cusps as in MPEG 22171. A scanning electronic microscope photo shows two flattened cusps, both with a series of micro cusps in “fetal” teeth of MPEG 22172 ( Fig. 16 View FIGURE 16 a). In both specimens lower jaw “fetal” teeth are in a single row, different from the multiple rows of Caecilia gracilis (MZUSP 57070).
Color. In life, M. taylori is pink ( Fig. 17 View FIGURE 17 ), purple or pale lavender, and this range of colour may occur in individuals of a single population. Venter and lateral surfaces slightly lighter than dorsum along entire body; area surrounding vent, mandible and part of head are paler. Nussbaum & Hoogmoed (1979) provided a slightly different description but pattern here described was observed in paratype RMNH 15165B. Dark color of dorsum extends laterally and ventrally in primary grooves of many specimens. Preserved specimens grayish or brownish, some with unpigmented blotches. Nussbaum & Hoogmoed (1979) reported paler spots posteriorly on the holotype.
Males Females
Variation. Males have wider and longer heads than females, consequently all measures related to HW and HL are larger also in males ( Table 10 View TABLE 10 ). A similar dimorphism was found in Brasilotyphlus guarantanus ( Maciel et al., 2009) . Nothing is known about the ecology and natural history of this species, we can not make inferences about the cause of the dimorphism in head dimensions. Sexual dimorphism in head size was also reported for the African gymnophion Schistometopum thomense (Bocage, 1873) , in which males have greater head width and head length than females ( Nussbaum & Pfrender, 1998). Delêtre & Measey (2004) tried to determine the cause of sexual dimorphism in S. thomense , rejecting an ecological hypothesis and postulating several hypothesis of sexual selection.
Geographic variation is not evident ( Table 11 View TABLE 11 ). The largest single-locality sample encompasses practically the entire species range in number of primary and secondary annuli. Large differences in sample sizes among populations prevent statistical analysis to test for geographic differences.
Natural history. Nussbaum & Hoogmoed (1979) reported specimens of M. taylori from decayed logs in forest, one specimen found together with an individual of A mphisbaena vanzolinii Gans, 1962. Other collectors have reported similar microhabitats in Caxiuanã (M.S. Hoogmoed, pers. obs.; J. Gomes, pers. obs.). Caldwell & Araújo (2005) at Belo Monte, Rio Xingu dug specimens of Microcaecilia sp. (which we suppose to be M. taylori , see below) from rotten palm roots in a sandy-loamy soil and from mounds of soil at the base of an unidentified species of palm tree. At Curua-Una these same authors collected a specimen from the stomach of the elapid snake Micrurus lemniscatus (L.), which was collected at night near pools in a road through forest.
Remarks. The lack of records of Microcaecilia taylori in Brazilian Guiana causes a disjunct distribution pattern, with most localities south of the Amazon and only the type locality at the border of Suriname and Brazil north of the Amazon ( Fig. 13 View FIGURE 13 ). However, because we found no morphological characters that separate these populations, we consider them all to be M. taylori .
Caldwell & Araújo (2005) reported Microcaecilia sp. from two localities in Pará south of the Amazon, viz. Belo Monte, Rio Xingu and Curua-Una. In a remark under “General comments it is stated that this is an undescribed species according to a pers. comm. of R. Nussbaum. Although we were not able to examine this material, we tentatively consider these specimens to be M. taylori on the basis of the picture published, and on the fact that from one locality studied by Caldwell & Araujo (2005) (Belo Monte, Rio Xingu) we have examined several recently collected specimens referable to this species.
Although the type specimens of M. taylori have no SG ( Nussbaum & Hoogmoed, 1979), we found this character to be much more variable (0–21) in our large series. Because there are no other differences between specimens, and because the number of SG in other species is known to be variable, we have identified all these specimens as M. taylori .
Distribution. Brazil (state of Pará, south of the Amazon) and Surinam (area of Sipaliwini savanna, 7 km from the Brazilian border) ( Fig. 13 View FIGURE 13 ).
populations of Microcaecilia taylori . Type data from literature ( Nussbaum & Hoogmoed, 1979), except for RMNH 15165B. WNC 3.5 (1) 2.8–5 2.4–5.2 3.7 (1) 3.7–4.4 (2) 3.8±1.7 (79) 4 ± 1.4 (16)
continued next page Type locality Parauapebas Caxiuanã Tucuruí Belo Monte Diagnosis. Maximum known TL 450 mm. Tentacles small, laterally positioned, near margin of mouth, closer to eye than nostril or corner of mouth; visible or not from above. Tentacles closer to eyes than nostrils. Nostrils subcircular, may or not be visible from above. Tongue anteriorly attached to mandibular mucosa, no narial plugs. Dentition in three series; splenials absent. Dermal and subdermal scales absent. Terminal shield large; posteriormost annuli anterior to vent. Secondary annuli absent. One species in Amazonian Brazil.
TABLE 10. Statistic results from analysis of sexual dimorphism of Microcaecilia taylori.
| Variables Range mean ± SD and (N) | Range Mean ± SD and (N) | ANCOVA |
|---|---|---|
| HW 2.8–4.7 3.9 ± 0.5 (46) | 2.9–4.7 3.7 ± 0.4 (54) | F = 15.385 P = 0.000 |
| HL 4.3–7 5.6 ± 0.6 (45) | 4.2–6.7 5.3 ± 0.5 (54) | F = 18.419 P = 0.000 |
| TN 1.4–2.6 1.9 ± 0.3 (45) | 1.4–2.6 1.7 ± 0.2 (54) | F = 18.728 P = 0.000 |
| TCM 0.8–2 1.3 ± 0.3 (45) | 0.8–1.6 1.2 ± 0.2 (53) | F = 12.243 P = 0.001 |
| NN 1–1.7 1.3 ± 0.2 (45) | 0.9–1.6 1.2 ± 0.1 (53) | F = 10.021 P = 0.002 |
TABLE 11. Morphometric (in mm) and meristic data (range, mean ± standard deviation and sample size in parenthesis) among
| Type locality | Parauapebas | Caxiuanã | Tucuruí | Belo Monte | |
|---|---|---|---|---|---|
| TL | 122–172 148.3±20.4 (3) | 77–225 168.7±45.5 (100) | 84–213 168.6 ± 52.3 (16) | 177.3 (1) | 173–200.2 (2) |
| HW | 3.3–4.5 3.7±0.5 (3) | 2.6–4.7 3.7±0.8 (101) | 2.7–4.7 3.8 ± 1 (17) | 3.5 (1) | 3.5–4.3 (2) |
| HL | 4.5–6.3 5.6±0.7 (3) | 3.7–7 5.3±1.1 (101) | 4.1–6.4 5.3 ± 1.3 (17) | 4.8 (1) | 5.1–5.8 (2) |
| HH | 2.5 (1) | 1.8–4.2 2.8±0.7 (100) | 1.8–3.6 2.7 ± 0.8 (17) | 2.9 (1) | 2.3–3.4 (2) |
| BW | 3.6–5 4.2±0.5 (3) | 2.3–5.9 4.2±2.1 (69) | 2.2–5.3 4.2 ± 1.5 (16) | 4 (1) | 3.3–5 (2) |
| BH | 3.3 (1) | 2.1–5.1 3.4±1.7 (69) | 1.8–4.9 3.6 ± 1.3 (16) | 3.4 (1) | 2.6–3.7 (2) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Microcaecilia taylori Nussbaum & Hoogmoed, 1979
| Maciel, Adriano O. & Hoogmoed, Marinus S. 2011 |
Microcaecilia taylori
| Caldwell 2005: 6 |
| Frost 1985: 623 |
| Nussbaum 1979: 245 |