Caecilia marcusi Wake , 1985
|
publication ID |
https://doi.org/ 10.5281/zenodo.203509 |
|
DOI |
https://doi.org/10.5281/zenodo.5635404 |
|
persistent identifier |
https://treatment.plazi.org/id/3E0BA842-4D51-2A58-BD96-D48D1C44F809 |
|
treatment provided by |
Plazi |
|
scientific name |
Caecilia marcusi Wake , 1985 |
| status |
|
Caecilia marcusi Wake, 1985 View in CoL
Caecilia marcusi Wake, 1985: 215 View in CoL ; Reichle & Köhler, 1996: 208; De la Riva, 1990: 300; De la Riva et al., 2000: 52; Köhler, 2000: 58; Cortez et al., 2004; Glaw & Franzen, 2006:156; Frost, 2008.
Caecilia mertensi Faria & Mott, 2011: 53 View in CoL .
Diagnosis. Maximum known TL 510 mm. TL. PA 139–157; SG 25–47. Eye visible in open socket. Dermal scales present; scale inception between annulus 22 and 95; at most four rows of scales per fold. Terminal shield present.
Description. TL 31.7–51 times (41.7 ± 5.4; n = 32) BW. Head slightly narrower than body. Snout projecting distinctly beyond mouth (e.g. 2.5 mm in MPEG 25178). Nuchal grooves distinct dorsally, laterally and ventrally in most specimens, except third nuchal groove which ventrally is incomplete; second nuchal collar ventrally partially fused with first primary fold; a dorsal transverse groove on each collar, shorter and less distinct on first; there may be a ventral transverse groove on first collar in some specimens (e.g. UFMT 6290); some specimens may have indistinct collars or collars that are only partially distinct. Body subcylindrical, slightly wider than deep. Width along body may vary slightly.
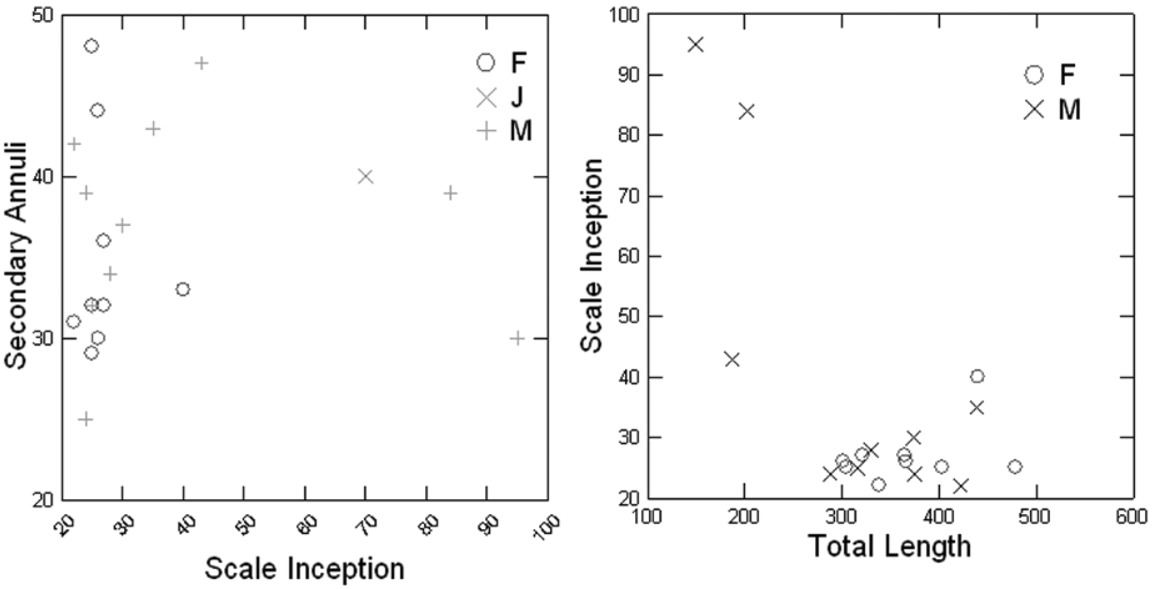
Primary annular grooves incomplete, interrupted dorsally and ventrally, except for posteriormost primary grooves (at most eight, 4.2 ±1.6; n = 29) which are complete. From general region where SG appear, to level of vent, annular grooves are dorsally complete. At most three secondary grooves complete (0.4 ± 0.7; n = 31). Unsegmented terminal shield posterior to vent, depressed in some specimens. No annular grooves posterior to vent. Vent transverse, may be slightly irregularly shaped; AD 9–17 (12.5 ± 5.4; n = 26); generally an approximately similar number in posterior and anterior edge of the vent (e.g. seven anterior, six posterior in MPEG 25178). Paired anal papillae found in only three of 14 males and one of 17 females in a sample of 32 specimens. SI between annulus 22 and 95; at most four rows of dermal scales per fold. Dermal scales and number of rows per fold increasing in size and in number, from anteriorly to posteriorly. No strong relation between SI and TL, but the smallest specimens have a higher SI value than the others ( Fig. 6 View FIGURE 6 ). No apparent relation between number of SG and SI ( Fig. 6 View FIGURE 6 ) as in Microcaecilia taylori (see below). Tongue not completely attached anteriorly to mandibular mucosa. PMT maximally 19 with notable variation in size, posterior maxillary teeth smaller, extending posteriorly of the level of choanae as for PPT series. PPT maximally 20, posterior teeth smaller. teeth smaller than PMT. DT maximally 17, approximately same size as PMT; posterior teeth smaller. ST maximally four, approximately same size as PPT.
Colour. Colour in life grayish blue or dark lavender. Lateral and ventral surfaces of the body are slightly paler than dorsum. Dark colour of dorsum extends laterally and ventrally in primary grooves of most specimens. Tip of tongue blackish, darker than rest. Some specimens have whitish venter with scattered dark blotches of irregular size and shape of approximately dorsum colour.
Variation. For variation in measurements and meristic characters see Table 4 View TABLE 4 .
populations of Caecilia marcusi and the holotype (and only known specimen) of C. mertensi . Type data from literature ( Taylor,
1973; Wake 1984), except for ZSM 82/1982, a paratype of C. marcusi . Numbers in bold represent recent counts for PMT, PPT,
and DT provided by A. Leviton (CAS).
continued next page Distribution. Bolivia (Cochabamba and El Beni); Brazil (states of Acre, Mato Grosso, and Rondônia) ( Fig. 7 View FIGURE 7 ).
Remarks. Taylor (1973) described Caecilia mertensi from a single specimen with the doubtful locality Seychelles Isle but supposed that “South America ” was the type-locality considering the distribution of the genus Caecilia . Wake (1985) described C. marcusi based on four specimens collected in the District of Cochabamba, Bolivia, and compared them with the holotype (and only reported specimen) of the superficially similar C. mertensi . Wake (1985) considered the specimens from Bolivia as a new taxon that differed from the holotype of C. mertensi in (1) head shape, (2) size and position of naris, (3) teeth counts and (4) colour. We checked each of these purported differences by examining one of the types of C. marcusi and comparing it with photographs of the holotype of C. mertensi plus additional observations of this specimen made by A. Leviton (CAS):
(1) Wake (1985) considered C. marcusi to have a “proportionally longer, narrower head”. Comparisons of morphometric data in bivariate plots of head measures versus total length of the type specimens of C. marcusi , the holotype of C. mertensi , and specimens recently collected in Brazil showed no clear difference among these specimens ( Fig. 8 View FIGURE 8 ).
(2) Wake (1985) reported C. marcusi as having a “larger, more dorsal naris” but we are unable to assess this because the holotype of C. mertensi has been damaged since Taylor’s (1973) description.
(3) Tooth count variation may result at least in part from difficulties in counting teeth. In a paratype of C. marcusi (ZSM 82/1982), we counted more PMT and DT than Wake (1985) (respectively 18 and 16, instead of 12 and 14). Tooth counts for the holotype of C. mertensi made by A. Leviton are substantially lower than those presented by Taylor (1973) and Wake (1985) (18 premaxillary-maxillary instead of 21; 12 prevomerine-palatine teeth instead of 23, and 16 dentary teeth instead of 24). These values fall clearly within the range of variation of Brazilian and Bolivian specimens of C. marcusi ( Table 4 View TABLE 4 ), but the mouth of the holotype of C. mertensi has become badly damaged (and no splenials could be found). Our counts for C. mertensi based on recent photographs agree with Leviton’s, but Taylor’s counts also seem reasonable based on his ( Taylor, 1973) photographs of the specimen at that time.
(4) Colour and colour pattern may vary among live or preserved specimens of caecilians, and we are less convinced than Wake (1985) that this is indicative of a specific difference. The photo of the holotype (ZSM 79/1982) of C. marcusi published by Glaw & Franzen (2006) does not show the marked difference between dorsal and ventral colour mentioned by Wake (1985). It seems there is a longitudinal furrow on the sides which might give the impression of a line, but in our opinion it is not a demarcation of dorsal and ventral colours. Neither could we observe a pronounced line of demarcation of dorsal and ventral colours in the paratype that we observed directly (ZSM 82/1982), in which the dorsal colour continues to the sides of the belly in the primary grooves. This agrees more or less with the description provided by Wake (1985), but we only could observe that dorsal and ventral colour abut clearly between the grooves. In our recently collected specimens from Brazil we observed considerable variation: some have distinctly darker backs than bellies, in others the colour difference is not that strong, but none of them shows a sharp demarcation between the colours, the transition is gradual or the dark dorsal colour descends along the primary grooves.
We conclude that there are no substantial differences between C. mertensi and C. marcusi other than a different maximum tooth number in three series (that can not be checked easily now in C. mertensi ). However, we did not directly observe the holotype of C. mertensi and it has no locality data, and so we refrain from placing C. marcusi in the synonymy of C. mertensi , and we refer the specimens from Brazilian populations to C. marcusi .
TABLE 4. Morphometric (in mm) and meristic data (range, mean ± standard deviation and sample size in parenthesis) of four
| C. mertensi C. marcusi type types | Acre | Mato Grosso | Rondônia | |
|---|---|---|---|---|
| TL | 480 (1) 385–510 449±44.9 (4) | 355 (1) | 149–445 331.4±71.7 (28) | 440 (1) |
| HW | 9 (1) 6.8–7.9 7.2±0.4 (4) | 6.4 (1) | 3.9–7.2 5.7±0.8 (28) | 6.8 (1) |
| HL | 12 (1) 11.2–13.3 11.9±0.8 (4) | 9.1 (1) | 5.6–10.1 8.4±1.2 (28) | 10.6 (1) |
| HH | 4.3 | 4.6 (1) | 2.6–5.6 4.2±1.9 (21) | 5.4 (1) |
| BW | 8.6–10.4 9.4±0.7 (4) | 7.5 (1) | 4.7–10.6 7.9±2.8 (25) | 9.3 (1) |
| BH | 7.3 (1) | 7 (1) | 4 –10.4 6.9±2.8 (24) | 8.4 (1) |
| WNC | 8.2 (1) | 6.6 (1) | 4.4–8 6.6±1.9 (26) | 7.9 (1) |
| WTR | 8.6 (1) | 6.7 (1) | 4.1–9.8 6.9±1.9 (27) | 8.3 (1) |
| WV | 7.2 (1) | 5 (1) | 2.8–7.3 4.9±1.4 (27) | 5.6 (1) |
| EE | 5.7 (1) 5.2–6.2 5.5±0.3 (4) | 4.4 (1) | 2.6–4.9 3.9±0.9 (27) | 4.5 (1) |
| EN | 3.5 (1) | 2.6 (1) | 1.7–3.2 2.6±0.6 (27) | 3.5 (1) |
| ET | 4 (1) 3.5–3.9 3.6±0.1 (4) | 2.8 (1) | 1.8–3.2 2.6±0.6 (27) | 3.6 (1) |
| EJA | 4.3 (1) | 3 (1) | 1.6–3.7 2.6±0.6 (27) | 3.4 (1) |
| EMM | 1.6 (1) | 1.3 (1) | 0.8–1.4 1.1±0.2 (27) | 1.5 (1) |
| SP | 1.6 (1) 1.9–2.4 2.1±0.1 (4) | 2 (1) | 1.2–2.5 1.9±0.2 (28) | 2.5 (1) |
| TT | 3.4 (1) | 3.1 (1) | 2.1–3.7 2.9–0.4 (28) | 3 (1) |
| TN | 1.9 (1) 1.3–2 1.7±0.2 (4) | 1.2 (1) | 0.7–1.4 1±0.2 (28) | 1.3 (1) |
| TCM | 6.9 (1) | 5.7 (1) | 3.4–6.5 5.1±0.7 (28) | 6.2 (1) |
| TMM | 1.2 (1) | 0.7 (1) | 0.5–1.2 0.9±0.1 (28) | 1.3 (1) |
| NN | 3.5 (1) 2.7–3.6 2.1±0.3 (4) | 2.3 (1) | 1.4–2.6 2.1±0.3 (28) | 2.3 (1) |
| NCM | 7.7 (1) | 6 (1) | 3.6–7.1 5.4±0.8 (28) | 6.4 (1) |
| NMM | 2.3 (1) | 1.9 (1) | 1.2–2.3 1.7±0.2 (28) | 2.1 (1) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Caecilia marcusi Wake , 1985
| Maciel, Adriano O. & Hoogmoed, Marinus S. 2011 |
Caecilia mertensi
| Faria 2011: 53 |
Caecilia marcusi
| Glaw 2006: 156 |
| Kohler 2000: 58 |
| Reichle 1996: 208 |
| Wake 1985: 215 |