Squalus clarkae, Pfleger & Grubbs & Cotton & Daly-Engel, 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4444.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:5B20789A-15E5-4451-BF80-D014E6B809BD |
|
DOI |
https://doi.org/10.5281/zenodo.5966645 |
|
persistent identifier |
https://treatment.plazi.org/id/3E0FEE3A-F859-FFE7-71C3-FBA79E98FB43 |
|
treatment provided by |
Plazi |
|
scientific name |
Squalus clarkae |
| status |
sp. nov. |
Squalus clarkae sp. nov.
Gulf Dogfish/Genie’s Dogfish
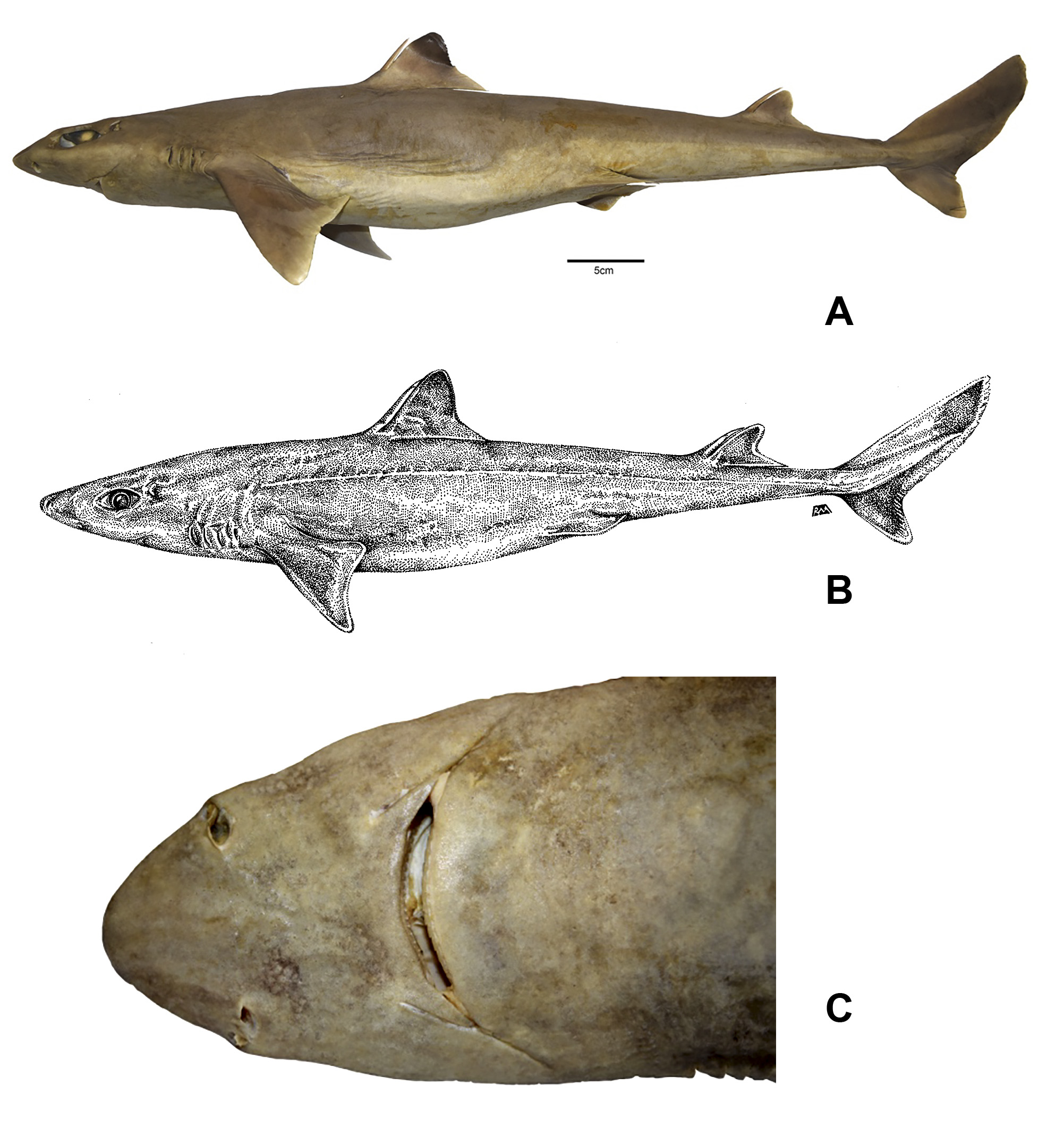
Description: Morphology and meristics. Squalus clarkae sp. nov. is a relatively large dogfish shark with a fusiform body, short head, and an interdorsal space ranging from 24.6% to 25.8% of TL. Head length is 21.2– 21.9% TL, and head width measures 1.08–1.2 times width at the mouth and 1.73–1.8 times width at the nostrils. Head height is about half the length of the head. Pre-vent length is 50.1–51.1% of the TL. S. clarkae sp. nov. is relatively short snouted, with a prenarial length 0.64–0.69 times mouth width and 1.32–1.59 times eye length. Interorbital space is equal to that of the mouth width, and prenarial length is 0.52–0.55 times preoral length. Eye length is 1.60–1.88 times eye height. Spiracle length is 1.0–1.2% of the TL of the animal. Fifth gill slit height is slightly larger than (1.2–1.4 times) first gill slit height. Mouth width is 0.78–0.82 times the pre-oral length. Innernostril labial furrow space is 1.54–1.89 times labial furrow length, and preoral length is 1.9–2.1 times the internarial space. Pre-first dorsal fin length 30.5–31.4% of the TL of the animal. First dorsal fin length measures 1.92–2.10 times first dorsal fin height. First dorsal fin length is approximately equal to that of the second dorsal fin (0.98–1.08 times); however, the height of the first dorsal fin is 1.50–1.68 times the height of the second dorsal fin. Pre-second dorsal fin length is 60.8–63.0% of the TL of the animal. Second dorsal fin length 2.97–3.28 times the second dorsal fin height. Second dorsal spine length is 1.05–1.17 times the length of the first dorsal spine, and second dorsal spine base width is 0.78–1.02 times the first dorsal spine base width. The pectoral fin inner margin was 7.0–8.4% of the TL, and pectoral anterior margin was 2.49–2.81 times the pectoral fin base length. Pelviccaudal space is 1.05–1.12 times the pectoral-pelvic space, and 1.24–1.28 times the prepectoral length of the organism ( Table 1).
The dermal denticles are small in size (approximately 250–300 µm length in adults) and generally triangular in shape. Each denticle has three ridges that run the length of the scale, with the center ridge most prominent, and terminates in a tricuspidate margin ( Figure 1 View FIGURE 1 ). The teeth are similar in appearance in the upper and lower jaw, each with a strongly oblique, laterally–directed cusp without serrations or cusplets. Each tooth has a broad base and a deep lateral notch below the cusp ( Figures 2 View FIGURE 2 and 3 View FIGURE 3 ). Total number of teeth range 27–29 in the upper jaw and 23–24 in the lower jaw, with no observed differences in dental formula or tooth morphology between sexes. Total precaudal vertebrae number between 87 and 90 centra, including 44–45 monospondylous centra and 43–45 diplospondylous centra. Caudal vertebral centra number 24–27 and total centra number 111–114.
Based on the morphometrics and description of the Squalus mitsukurii holotype in Last et al. (2007a), S. clarkae sp. nov. is overall longer-bodied, with a precaudal length ranging 79.7–82.9% TL compared with 78.2– 79% TL, dorsal-caudal space 11.4–18.5% TL compared with 9.8–11.2% TL, and pre-second dorsal length 60.8– 63.0% TL as compared with 58.6–61.2% TL. The eyes are closer-set, with the interorbital space of S. clarkae sp. nov. ranging 7.0–8.0% TL as opposed to 7.9–8.4% TL in Squalus mitsukurii . In addition, the snout of S. clarkae sp. nov. is substantially shorter than S. mitsukurii , with the preorbital length ranging 7.0–7.2% TL in S. clarkae sp. nov. and 7.3–7.9% TL in S. mitsukurii , preoral length 8.7–9.5% TL in S. clarkae sp. nov. and 9.4–10.8% TL in S. mitsukurii , and prenarial lengths of 4.5–5.1% TL in S. clarkae sp. nov. and 5.0–5.5% TL in S. mitsukurii .
S. clarkae sp. nov. exhibited a shorter first dorsal base length ranging 6.1–6.8% TL, while S. mitsukurii dorsal base lengths were 7.8–8.3% TL. The first dorsal anterior margin was also shorter in S. clarkae sp. nov., ranging 9.0–10.2% TL, and 10.5–12.0% TL in S. mitsukurii . The second dorsal posterior margin was slightly larger in S. clarkae sp. nov. than that of S. mitsukurii , ranging 5.0–5.5% TL and 4.1–5.2% TL, respectively. As in the first dorsal, the second dorsal base length is smaller in S. clarkae sp. nov. (5.8–7.5% TL) than in S. mitsukurii (7.2– 9.2% TL), and the second dorsal anterior margin is shorter in S. clarkae sp. nov. (8.3–10.2% TL) than in S. mitsukurii (10.2–10.7% TL). The caudal fin is shorter in S. clarkae sp. nov., with caudal fork length (8.4–9.0% TL) compared with S. mitsukurii (9.2–10.3% TL), as well as at the dorsal caudal margin ( S. clarkae sp. nov. = 18.5– 20.9% TL; S. mitsukurii = 21.2–22.6% TL; Table 1).
……continued on the next page
Morphological comparison between S. clarkae sp. nov. and the four new species described from the southwest Atlantic, Squalus lobularis Viana, 2016 , S. bahiensis Viana, 2016 , S. albicaudus Viana, 2016 , and S. quasimodo Viana, 2016 ( Viana et al. 2016), also yielded a number of substantial differences. The southernmost species, S. quasimodo (31o S), has a long, pronounced caudal keel extending from D2 to the caudal pit, while S. clarkae sp. nov. has a much smaller keel. S. quasimodo also has a pronounced dorsal “hump” which is absent in S. clarkae sp. nov.. The dorsal caudal lobe is much wider than in S. clarkae sp. nov., with a broadly rounded tip, and the posterior pectoral margin is approximately even with the fin spine in S. quasimodo but anterior to the spine in S. clarkae sp. nov.. Dorsal and pectoral fins are also significantly larger in S. quasimodo than in S. clarkae sp. nov..
Squalus lobularis (20o S) is the most morphometrically similar to S. clarkae sp. nov., overlapping in nearly every character. Squalus clarkae sp. nov. has a relatively longer interdorsal space (24.1–25.4% TL) than S. lobularis (21.9–24.6% TL) and the inner margin of the pectoral fin is larger in S. lobularis (9.2–11.1 % TL) than S. clarkae sp. nov. (7.0–8.9% TL). As the name implies, S. lobularis has rounded (lobular) dorsal fins, unlike S. clarkae sp. nov., which has pointed dorsal fins.
Squalus bahiensis (15o S) is morphologically similar to S. mitsukurii and many of the characters that separate S. clarkae sp. nov. from S. mitsukurii also separate it from S. bahiensis . Squalus clarkae sp. nov. has a shorter snout than S. bahiensis as described by the preorbital length (7.0–7.3% TL and 7.3–7.9% TL, respectively) and preoral length (9.0–9.5% compared to 9.9–10.5% TL, respectively). Squalus clarkae sp. nov. also has an overall smaller first dorsal fin than S. bahiensis : the first dorsal fin length is 11.7–12.9% TL in S. clarkae sp. nov. compared to 12.8–13.8% TL in S. bahiensis , and the first dorsal anterior margin is 9.0–10.2% TL in S. clarkae sp. nov. compared to 10.3–10.5% TL in S. bahiensis . The first dorsal fin spine is longer in S. clarkae sp. nov. (3.4– 4.2% TL) than in S. bahiensis (2.9–3.0% TL). Squalus bahiensis also has rounded pectoral tips compared with S. clarkae sp. nov. ’s pointed ones, a more rounded snout, and markedly different tooth morphology: short, upturned, laterally directed cusps, with mesial cutting edges “conspicuously convex” in S. bahiensis compared with the longer, straight mesial cutting edges with laterally-directed cusps in S. clarkae sp. nov..
Though Squalus albicaudus (12o S) is the closest geographically to the GoM of the four recently-described species, its denticles are markedly different from S. clarkae sp. nov. and actually place this species in the “ megalops ” complex: slender, unicuspidate, non-overlapping, and lanceolate as opposed to wider, tricuspidate, overlapping, and rhomboid in S. clarkae sp. nov.. Squalus albicaudus is morphologically very similar to S. cubensis , a sympatric species with S. clarkae sp. nov.. Squalus albicaudus has a shorter prenarial length ( PRN) than S. clarkae sp. nov. (4.3–5.0 versus 5.0–5.5% TL), and pointed free rear pectoral tips versus the rounded ones belonging to S clarkae sp. nov.. Further, three of the four new species described by Viana et al. (2016), with the exception of S. albicaudus , lack the diagnostic caudal “bar” that is found at the fork of the caudal fin in S. clarkae sp. nov. ( Figures 4 View FIGURE 4 and 5 View FIGURE 5 ). While morphological differences between S. clarkae sp. nov. and other members of the genus are slight, this is not uncommon for species within the genus.
The six specimens used in the morphological descriptions of S. clarkae sp. nov. have been deposited as type specimens in either the Florida Museum of Natural History Ichthyology Collection or the Florida State University Coastal and Marine Laboratory Zoological Collection. These include the 71.2 cm female holotype (UF-239318, FSUCML-873), 55.5 cm male paratype (UF-239319, FSUCML-1017), 68.1 cm female paratype (UF-239320, FSUCML-874), 67.8 cm female paratype (FSUCML-875), 51.0 cm female paratype (FSUCML-1016), and 72.3 cm female paratype (FSUCML-1018).
Genetics. PCR of S. clarkae sp. nov., S. cubensis , and S. mitsukurii from Japan yielded 687 base pairs of COI (Acc # MG792161 View Materials – MG792175 View Materials ) and 585 base pairs of ND2 (Acc # MG792146 View Materials – MG792160 View Materials ), which were concatenated to create a 1272 base pair contig for analysis. jModeltest indicated the best substitution model was the HKY85, selected via Aikake Information Criteria. The maximum likelihood tree in Figure 6 View FIGURE 6 , which includes both genes and was consistent with Bayesian topology, shows clear separation between Japanese S. mitsukurii and S. clarkae sp. nov. from the Gulf of Mexico, with 98.6–99.7% bootstrap support and 1.00 posterior probability. Within-species divergence ( Table 2) ranged 0.079–0.786% (average = 0.487%) for Japanese S. mitsukurii , while within-species divergence ranged 0.079–0.734% (average = 0.330%) for S. clarkae sp. nov. in the Gulf of Mexico. Between-species divergence ( Table 2) for S. mitsukurii from Japan and S. clarkae sp. nov. showed a marked increase in scale, ranging 2.280–4.037% (average = 2.815%), which is consistent with other taxonomic studies within this genus ( Ebert et al. 2010; Last et al. 2007b; Ward et al. 2007). In addition, between-species divergence for S. cubensis and S. clarkae sp. nov. ranged 4.587–5.110% (average = 4.813%). Our results indicate that Japanese S. mitsukurii and S. clarkae sp. nov. from the Gulf of Mexico are more closely related than the cooccurring S. clarkae sp. nov. and S. cubensis ( Figure 6 View FIGURE 6 ), and that all three species of Squalus are differentiated from Cirrhigaleus australis with 7.076–8.440% sequence divergence (average = 7.485%). Also included in this analysis were samples from the Northwest Atlantic previously thought to be S. blainville , which has been commonly confused with S. mitsukurii in multiple ocean basins, despite the fact that the two were morphologically separated by Chen et al. (1979). Squalus blainville is currently believed to be solely restricted to the Mediterranean Sea and East Atlantic ( Ebert et al. 2010), a conclusion that is supported by the genetic data shown here, and taxonomic reexamination of that species is currently underway (A. Veríssimo, pers. comm.). Additionally, individuals of S. blainville from the West Atlantic were reclassified as S. clarkae sp. nov. based on genetic data presented later in this paper.
Genetic comparison between S. clarkae sp. nov. and the two Squalus species in the East Atlantic, S. megalops and S. blainville , as well as S. cf. mitsukurii in southern Brazil using the ND2 gene alone yielded wide genetic differences between species on par with other shark taxonomic studies. Maximum likelihood and Bayesian methods disagreed somewhat on the basal position of S. blainville in the Mediterranean relative to species in the West Atlantic, but all nodes were statistically well-supported, and the degree of between-species variation recovered here was congruent with other studies on Squalus using this gene ( Naylor et al. 2012; Veríssimo et al. 2010). Squalus clarkae sp. nov. was differentiated from all other putative species with bootstrap support of at least 95.4% and 0.920 posterior probability ( Figure 7 View FIGURE 7 ). Within-species variation between pairs of individuals was 0.000– 0.012% sequence divergence, while between-species distances ranged 1.050–4.866% ( Table 3). As with the analysis of concatenated mitochondrial genes, S. clarkae sp. nov. was shown to be unrelated to the co-occurring S. cubensis at ND2. Instead, this species appears most closely related to the DNA sequences from S. cf. mitsukurii obtained from Brazil ( Naylor et al. 2012), which may correspond to one of the new species described by Viana et al. (2016).
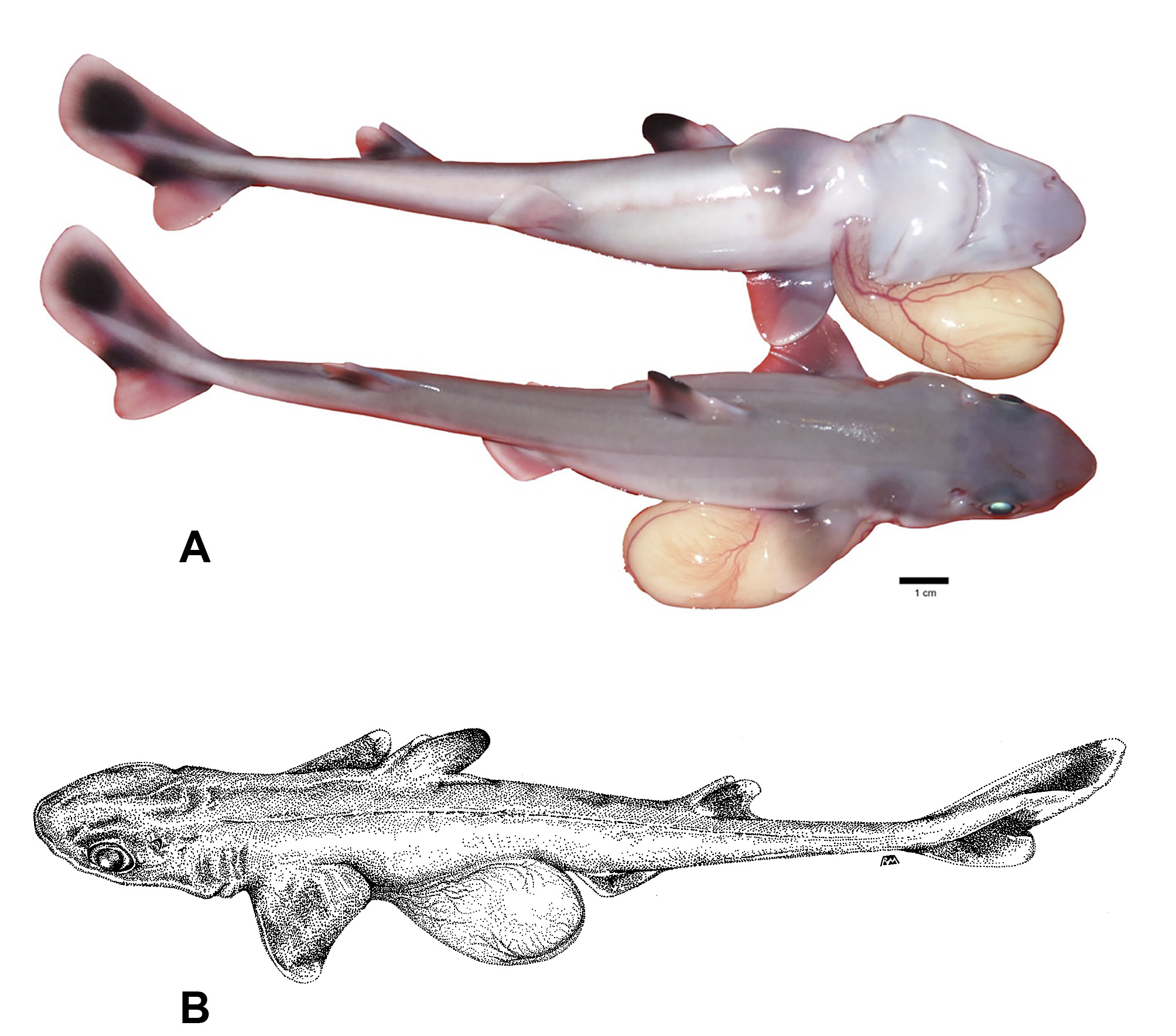
Color. The adult Squalus clarkae sp. nov. dorsum is gray throughout, ventral surface lighter gray to white ( Figure 4 View FIGURE 4 ), although it was noted that some individuals captured at night exhibit much darker tones, appearing nearly black dorsally and dark gray ventrally. This darker coloration fades to the typical coloration within minutes after capture. Adult pectoral fins fade to lighter gray on the trailing edges, while the juveniles exhibit white trailing edges ( Figure 5 View FIGURE 5 ). The first dorsal fin is lighter gray anteriorly near the base and darker gray posteriorly and black near the apex, a pattern that is substantially more pronounced in juveniles. Adults of this species exhibit a light posterior margin of the caudal fin that is broken by a dark bar that reaches the posterior edge of the fork of the caudal fin. In late-stage embryos and neonates, coloration of the caudal region is unique: the upper lobe of the caudal fin contains one large dark spot in the center, with white around the edges ( Figure 5 View FIGURE 5 ). The dark caudal bar that is retained in adults is pronounced, while the lower lobe is almost entirely white ( Figure 4 View FIGURE 4 ).
Distribution. Squalus clarkae sp. nov. were captured between 242 and 613 meters in the Gulf of Mexico. Capture locations for this study ranged from the West Florida Slope off of Tampa, Florida to the west side of the head of DeSoto Canyon off Louisiana. Specimens were captured at bottom temperatures ranging 6.92–13.77°C. The combination of the South Carolina S. blainville DNA sequences identified as S. clarkae sp. nov., plus the match with a single ND2 sequence from Naylor et al. (2012) from North Carolina, indicates that the range of S. clarkae sp. nov. extends from the Gulf of Mexico into the Northwest Atlantic. Results from the Southwest Atlantic show species-level differences between S. clarkae sp. nov. and S. cf. mitsukurii from Brazil, though the exact southern boundary of S. clarkae sp. nov. ’s range is unknown. No genetics were included in the recent morphological revision of S. mitsukurii from the southwest Atlantic ( Viana et al. 2016), so we are unable to confirm identification for the Brazilian sequences we obtained, apart from conclusively differentiating them from S. clarkae sp. nov.. The type locality of S. albicaudus , the northernmost species described in that revision, is located approximately 8,000 km from the reported range of S. clarkae sp. nov., a degree of geographic separation that likely precludes gene flow for this small-bodied shark.
Etymology. This species is named in honor of Dr. Eugenie Clark, a pioneer in the field of marine science broadly, and elasmobranch biology in the Gulf of Mexico specifically. Among her many accomplishments, Dr. Clark completed multiple submersible dives, led hundreds of research expeditions, and founded Mote Marine Laboratory in Southwest Florida which continues to conduct research in the Gulf of Mexico and abroad. Dr. Clark’s dedication to elasmobranch biology and marine conservation has served as a source of inspiration for countless scientists, including these authors. Her history of deep sea research and passion for fauna of the Gulf of Mexico inspired the etymology presented herein.
Common Name. Genie’s Dogfish
Remarks. Squalus clarkae sp. nov. shows many similarities to Squalus mitsukurii , but is morphologically, genetically, and geographically well-separated from type specimens in Japan, with a broad lack of overlap between the two species. Analysis also showed S. clarkae sp. nov. to be substantially different from congeners in the Gulf of Mexico, East Atlantic, and Brazil, indicating that the species is unlikely to extend south past the Caribbean, though the northern end of its range has not been delineated. This is consistent with recent work that has shown discrete, minimally-overlapping, and generally small species ranges for Squalus in the southwest Atlantic ( Viana et al. 2016). We conclude that S. clarkae sp. nov. is a novel species separate from S. mitsukurii , and have designated a holotype and several paratypes.
Future research on Squalus clarkae sp. nov. should focus on characterizing life history traits, as well as fully documenting the geographic distribution of the species, including comparing specimens of S. clarkae sp. nov. throughout its entire range. Sharks of the genus Squalus complex have a history of depletion ( Graham et al. 2001; Wilson & Seki 1994), and S. clarkae sp. nov. has been recorded as frequent bycatch in reef fish longline observer reports within the Gulf of Mexico ( Gulak & Carlson 2013). In the future, accurate life history and distribution data will be critical to forming effective management strategies for this newly described species, including population connectivity and taxonomic relationships with other members of the S. mitsukurii species complex.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |