Eugnosta azapaensis Vargas & Moreira
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3920.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:9FC2BFE2-92DE-473D-A896-D218FAFC55F8 |
|
DOI |
https://doi.org/10.5281/zenodo.6098557 |
|
persistent identifier |
https://treatment.plazi.org/id/3E2787A5-FF9F-FF9A-FF0E-FB18FD75FEFB |
|
treatment provided by |
Plazi |
|
scientific name |
Eugnosta azapaensis Vargas & Moreira |
| status |
sp. nov. |
Eugnosta azapaensis Vargas & Moreira View in CoL , sp. n
Figs. (1–8)
Type material. CHILE: Azapa, Arica, Chile, reared from galls on Baccharis salicifolia (Asteraceae) . Adults pinned, dried, and labeled HOLOTYPE: ♂, collected by H.A. Vargas in October 2011 ( MNNC). PARATYPES: 1♂, 1♀, collected by G.R.P. Moreira, G.L. Gonçalves & H.A. Vargas in January 2011; 1♂, collected by G.R.P. Moreira & H.A. Vargas in August 2012 ( MNNC); 2♂, collected by G.R.P. Moreira & H.A. Vargas in October 2012 ( IDEA).
Other specimens examined: Immature stages, same data as holotype, all collected by G.R.P. Moreira & H.A. Vargas, October 1–5, 2012; 10 larvae, preserved in 100% ethanol at -20°C ( LMCI 191-1); 8 larvae, fixed with Dietrich's fluid, preserved in 70% ethanol ( LMCI 191-4, 9,11); 6 pupae, fixed with Dietrich's fluid, preserved in 70% ethanol ( LMCI 191-5, 6, 8); 5 galls, fixed and preserved in 75% ethanol ( LMCI 191-13 to 17).
Diagnosis. Adults are mostly orange-brown, the wings without maculation, and with the costa and the termen of the forewing straight. The male genitalia bear large and well-sclerotized socii, one of the more diagnostic attributes of the genus ( Razowski 2011). The valvae are symmetrical, with the costal margin broadly concave. The phallus is cylindrical, with the vesica bearing two fused cornuti; one spiniform, circa 1/2 phallus length; the other short, about 1/3 length of larger cornutus. The phallus is curved, with the posterior end bent ventrally at 4/5 length. The pupa is obtect, darkish brown, with two anteriorly curved, strong spines dorsolaterally on A10. The larva is eruciform, hypognathous, with the head dark reddish brown, and the thorax and abdomen creamy white; the SV group of abdominal segment A1 unisetose. This is a cecidogenous species, inducing caulinar, fusiform galls on apical branches of Baccharis salicifolia (Asteraceae) , where larval development and pupation occur.
The male genitalia are similar to those of Eugnosta argentinae (Razowski, 1967) described from Santa Fe Province, Argentina. The cornutus in E. argentinae is slightly longer (about 3/4 phallus length) than in E. azapaensis ; the female genital structures of E. argentinae are undescribed, which precludes comparisons. The shape of the forewing costa and termen is convex in E. argentinae ; and the striking wing maculation of E. argentinae is absent in E. azapaensis . The male genitalia of E. azapaensis are also similar to those of Eugnosta ochrolemma (Razowski, 1986) described from Mexico. The external margins of the valvae are straight in E. ochrolemma . The female genitalia of E. ochrolemma have no signum on the corpus bursae. E. azapaensis induces similar galls to those of the Nearctic E. busckana ( Comstock, 1939) and E. beevorana , described by Comstock (1939, 1940) and Goeden and Ricker (1981). The adults of E. busckana and E. beevorana have maculate forewings. The larvae of E. azapaensis differ from those of E. busckana and E. beevorana by having reduced chaetotaxy (for example, two SV setae on A1, and only one SD seta on A1 and A7–8), and the pupal cremaster with a pair of strong spines, which are reduced to very small hooklets in E. busckana and E. beevorana .
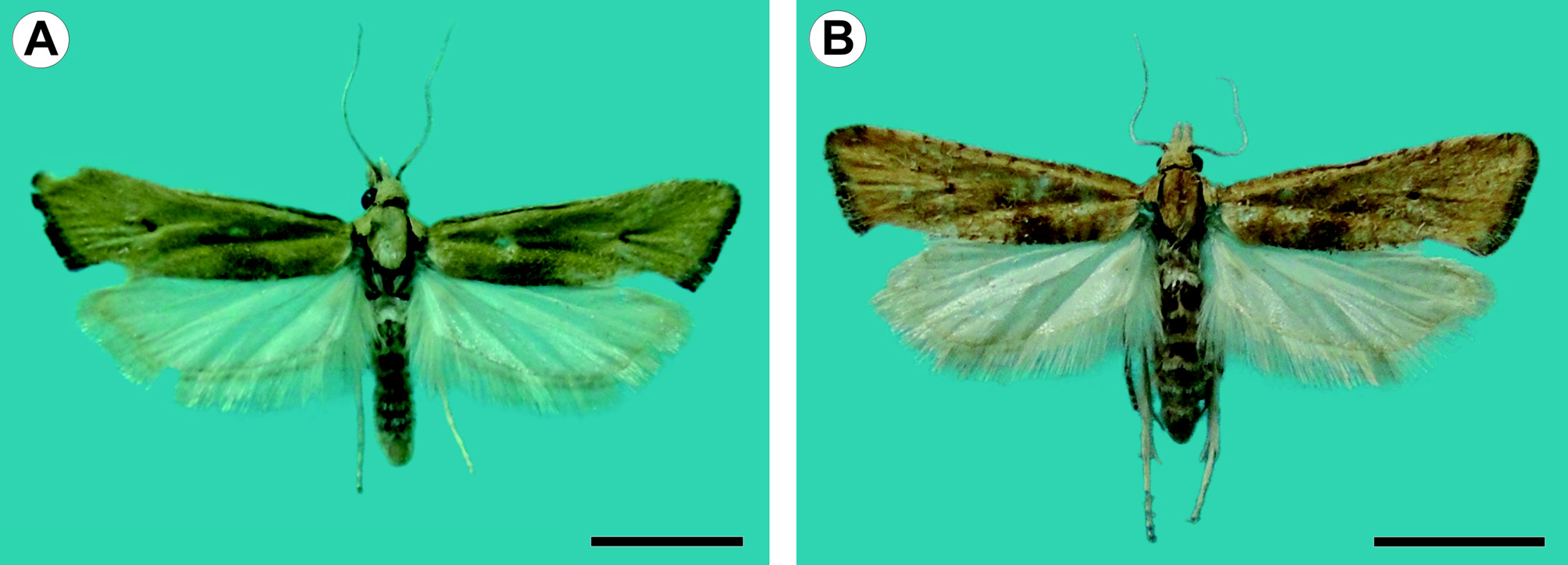
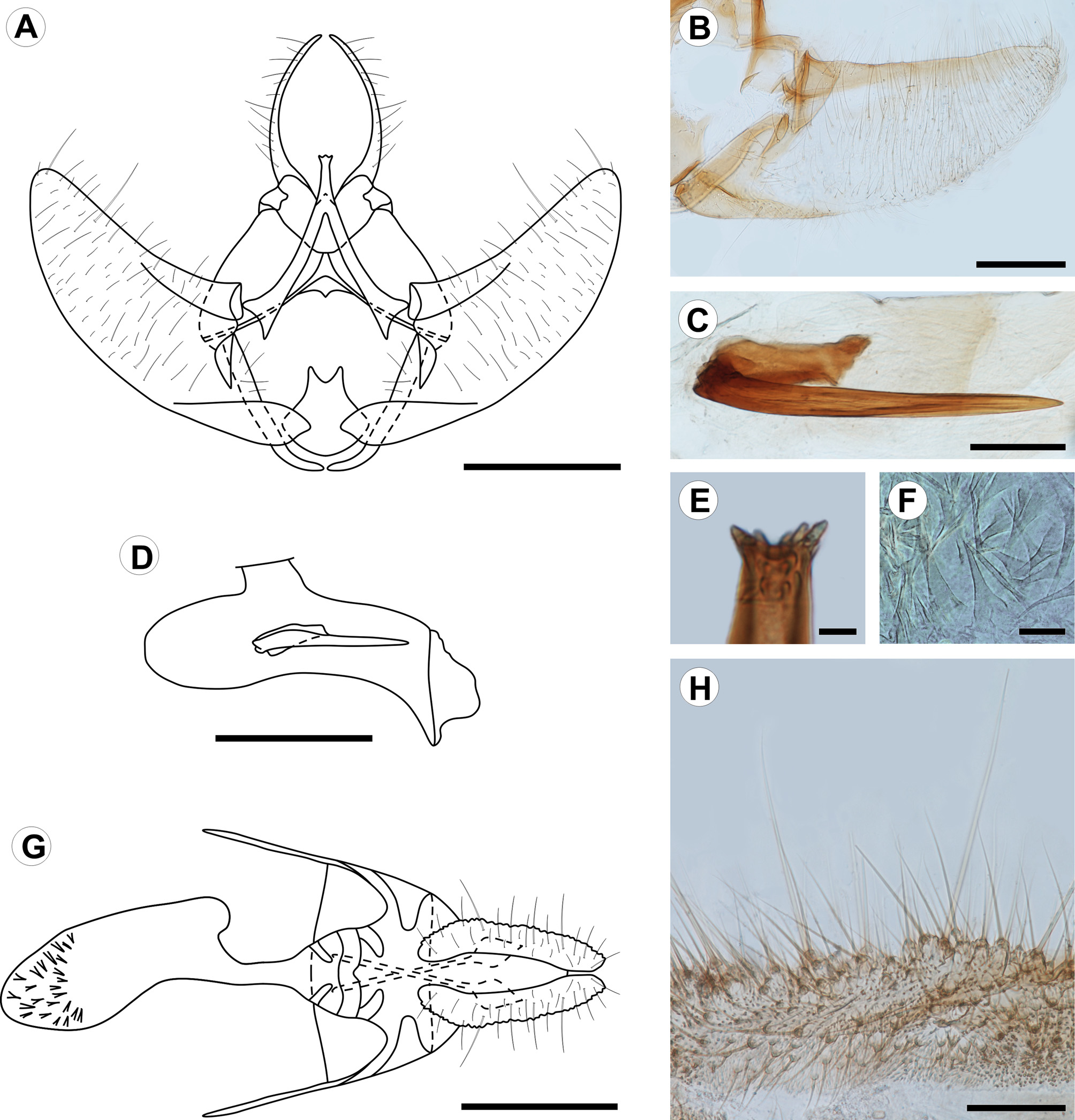
Description. Adult ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ): Male and female similar in size and color. Head: Mostly light orange-brown; vertex scales projecting upward, scales of front projecting downward; antenna filiform, nearly 2/3 length of forewing costa; scape and pedicel pale orange-brown; flagellum dorsally covered with pale orange-brown scales, ventral surface ciliate; labial palpus projecting forward, slightly upturned, length nearly 2.5 times diameter of compound eye, broadened at middle, pale orange-brown dorsally, grayish brown laterally and ventrally, second segment conspicuously longer than first and third segments, circa 1.5 times diameter of compound eye; proboscis short. Thorax: Pale orange-brown dorsally, pale whitish gray laterally, tegula with group of elongated, dark-brown scales arising from ventral arm and reaching base of frenulum; foreleg dark brown, with epiphyses arising at middle of tibia and reaching base of first tarsomere; midleg grayish brown, with one pair of spurs arising at apex; hindleg mostly whitish brown with some grayish-brown scales scattered on tibia and tarsus, with two pairs of spurs on tibia, one at middle, one at apex. Forewing length 7.9–9.2 mm (mean = 8.4, n = 3); dorsal surface mostly pale orange-brown, one small dark-brown discal spot, some dark-brown scales scattered along costa, one triangular grayish-brown spot arising at 1/3 length posterior margin with apex of spot reaching discal cell, fringe dark brown on distal margin; ventral surface mostly dark brown with many scattered grayish-brown scales. Hindwing pale whitish gray on both surfaces; fringe concolorous. Abdomen: Pale whitish gray with some scattered orange-brown scales. Male genitalia ( Fig. 2 View FIGURE 2 A–E) with uncus absent. Socii long, spinelike, strongly sclerotized, round apex, curved and converging distally. Tegumen broad, narrowing distally; saccus straight, lateral arms curved medially, not connected ventrally; transtilla well developed, sclerotized, medial portion arm-like, projected postero-dorsally, with small subapical projections; juxta with caudal margin circular, narrowed, with V-shaped incision on dorsal margin; valvae symmetrical, lightly sclerotized, costal margin broadly concave, ventral margin broadly convex, bases of costa and ventral margins more heavily sclerotized, apex broadly round, median and lateral surfaces covered with fine, elongated setae; phallus cylindrical, slightly longer than costa of valva, bases broadly round, bent ventrally at 4/5 length, apex with pointed ventral projection, vesica with strong, spinelike cornutus, slightly longer than 1/2 length of phallus, and one short cylindrical cornutus, about 1/3 length of larger cornutus, cornuti fused basally. Female genitalia ( Fig. 2 View FIGURE 2 F–H) with papillae anales flat, elongate, surface rough, with fine, elongate setae; posterior apophyses elongate, spinelike, about 3/4 length of papillae anales; anterior apophyses elongated, spinelike, similar in length to posterior apophyses; lamella antevaginalis a narrow band with posterior margin slightly cleft and anterior margin broadly convex; lamella postvaginalis with two slightly sclerotized diagonal patches; antrum mostly membranous, with two slightly sclerotized diagonal patches; ductus bursae cylindrical, membranous; corpus bursae mostly membranous, with group of short spinelike signa at apex; ductus seminalis located ventrally at middle of corpus bursae; ductus bursae ca. ½ length of papillae anales.
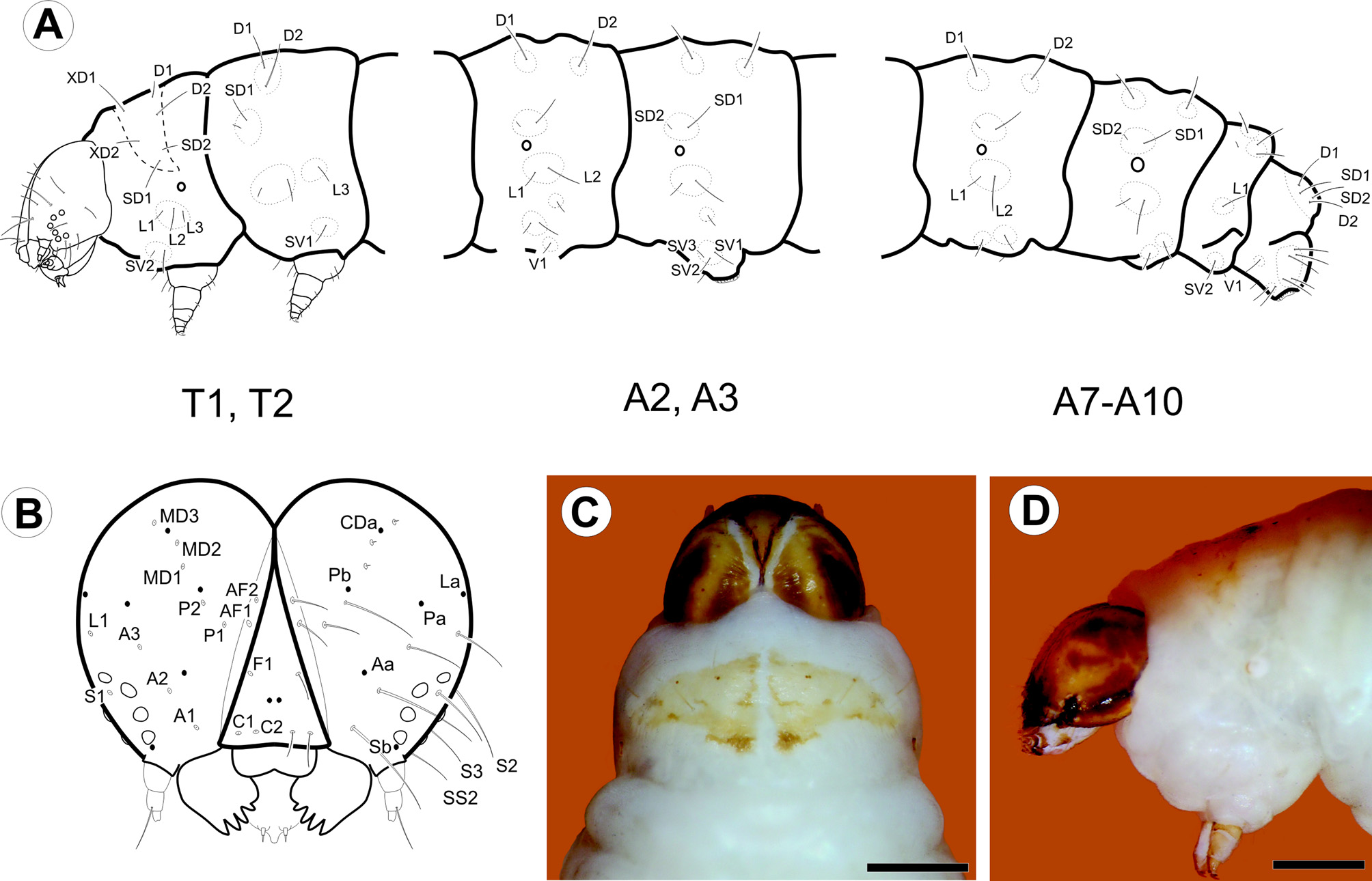
Last instar larva ( Figs. 3 View FIGURE 3 A–D, 4A–L, 8E): Eruciform, hypognathous, head dark reddish brown, thorax and abdomen creamy white, thoracic and abdominal setae on unpigmented pinacula. Head: Sub-spherical; six stemmata, arranged in semicircle on each side of head, posterodorsally to antenna; frontoclypeus triangular. Antennae three-segmented; first segment annular, short; second segment cylindrical, about two times length of first segment, bearing four sensilla: two long and filiform, two short and conical; third segment cylindrical, short, about 1/2 length and width of second segment, bearing two sensilla. Mouthparts of chewing type; labrum bilobed, with 12 filiform setae on outer surface; mandibles well developed, with five teeth on distal margin, and two short filiform setae on external surface; maxillae with palpus and galea well differentiated. Thorax: Prothoracic shield pale brown, slightly sclerotized, separated longitudinally by dorsal cleft into two triangular halves, one pair of circular spiracles laterally on prothorax. Abdomen: Prolegs with uniordinal crochets in circle on prolegs of A3–6, uniordinal crochets in transverse bands on prolegs of A10; anal shield slightly differentiated; anal fork absent. Chaetotaxy: Head ( Fig. 3 View FIGURE 3 A–B) with 17 pairs of setae, 3 pairs of microsetae and 7 pairs of pores. Adfrontal (AF) group bisetose; both setae arising near dorsal 1/4 of adfrontal suture; AF2 closer to dorsal angle of frontoclypeus than AF1. Frontal (F) group unisetose; F1 arising at middle of frontoclypeus, near adfrontal suture; Fa almost equidistant from F1 and C2. Clypeal (C) group bisetose; C1 closer to intersection of ventral margin of frontoclypeus and adfrontal suture; C2 dorsomedial to C1. Anterior (A) group trisetose; A1 between adfrontal suture and stemmata; A2 dorsolateral to A1; A3 ventrolateral to A2; Aa pore dorsomedial to A2. Posterodorsal (P) group bisetose; both setae near ecdysial line; P2 dorsolateral to P1; Pa pore greatly displaced laterally to P2; Pb pore dorsolateral to P2. Cephalodorsal (CD) group trisetose; CD1, CD2 and CD3 microsetae; CD2 slightly medially displaced from imaginary line between CD1 and CD3; CDa pore between CD2 and CD3. Lateral (L) group unisetose; L1 dorsolateral to A3; La pore posterodorsal to L1. Stemmatal (S) group trisetose; S1 almost equidistant from stemma 2 and stemma 3; S2 ventral to stemma 1; S3 posteroventral to stemma 6; Sb pore ventral to stemma 6. MGa pore posteroventral to S2. Substemmatal (SS) group trisetose, forming triangle between stemmata and hypostomal ridge. Prothorax ( Fig. 3 View FIGURE 3 A) with 12 pairs of setae (XD1, XD2, D1, D2, SD1, SD2, L1, L2, L3, SV1, SV2, V1), 3 pairs of microsetae (MV1, MV2, MV3). XD, D and SD groups bisetose, all included on dorsal shield. One pore between XD1 and D1, another pore between XD and XD1. L group trisetose, on single pinnacle extending slightly under the anterior potion of the spiracle. SV group bisetose, on single pinaculum dorsal to coxa. V group unisetose. MV group of microsetae anterior to coxa. Meso- and metathorax: 9 pairs of setae (D1, D2, SD1, SD2, L1, L2, L3, SV1, V1) and 6 pairs of microsetae (MD1, MSD 1, MSD2, MV1, MV2, MV3); D group bisetose, with the two setae on a single pinaculum; microseta MD1 anteroventral to pinaculum of D group; SD group bisetose, with the two setae on a single pinaculum; microsetae MSD1 and MSD2 on pinaculum anteroventral to that of SD group; L group trisetose, with L1, L2 on a single pinacuulm, L3 on a separate pinaculum; SV group unisetose, on a single pinaculum; V group unisetose; MV group of microsetae anterior to coxa. Abdomen ( Fig. 3 View FIGURE 3 B) with A1, 7, 8 each bearing 9 pairs of setae (D1, D2, SD1, SD2, L1, L2, L3, SV1, V1) and 2 pairs of microsetae (MD1, MV3). D bisetose, each seta on a different pinaculum; microseta MD anteroventral to pinculum of D1; SD group bisetose, with both setae on a single pinculum, SD2 extremely reduced; pinaculum of SD group slightly anteriorly displaced on A8; L group trisetose, L1 and L2 on the same pinaculum, L3 on a separate pinaculum; SV group unisetose; V group unisetose; microseta MV3 anterior to SV1. A2: 10 pairs of setae and 2 pairs of microsetae; similar to A1, but SV bisetose on a single pinaculum. A3–6: 11 pairs of setae and 2 pairs of microsetae; similar to A2, but SV group trisetose on proleg. A9: 6 pairs of setae and 2 pairs of microsetae; right and left D2 on a single pinaculum; D1 and SD1 on the same pinaculum; L, SV and V groups unisetose. A10: 13 pairs of setae; D and SD groups bisetose, with all setae on anal shield; remaining setae on proleg.
Pupa ( Fig. 5 View FIGURE 5 A–C, 6A–F, 8F, H): Obtect; integument darkish brown. Head with front slightly projected ventroanteriorly ( Fig. 5 View FIGURE 5 C, 6A), with group of four setae between compound eyes near posterior margin ( Fig. 5 View FIGURE 5 B, 6B); mouthparts partially exposed ventrally ( Fig. 5 View FIGURE 5 B, 6A–B); antennae ( Fig. 5 View FIGURE 5 B–C, 6A) arising anteriorly, projected ventroposteriorly at posterior margin of A5, surpassing forewing apex. Prothorax ( Fig. 5 View FIGURE 5 A,C) dorsally as a narrow transverse stripe between head and mesothorax; mesothorax ( Fig. 5 View FIGURE 5 A–C) broad, dorsally with broadly concave anterior margin, posterior margin sinuous, laterally represented by the forewings, which mostly conceal hindwings; metathorax ( Fig. 5 View FIGURE 5 A–B) with posterior margin almost straight in dorsal view; thoracic legs partially exposed ventrally. Abdomen ( Fig. 5 View FIGURE 5 A–C, 6A–D) with tergum of segment A3 with transverse row of straight spines arising near posterior margin and posteriorly projected; terga of segments A4–8 with two transverse rows of straight spines, posteriorly projected ( Fig. 6 View FIGURE 6 C); circular spiracles dorsolaterally on A3–7 ( Fig. 6 View FIGURE 6 D); two strong spines arising dorsolaterally on segment A10, curved anterodorsally ( Fig. 6 View FIGURE 6 E–F).
Etymology: The specific epithet is derived from the type locality, the Azapa Valley, northern Chilean Atacama Desert.
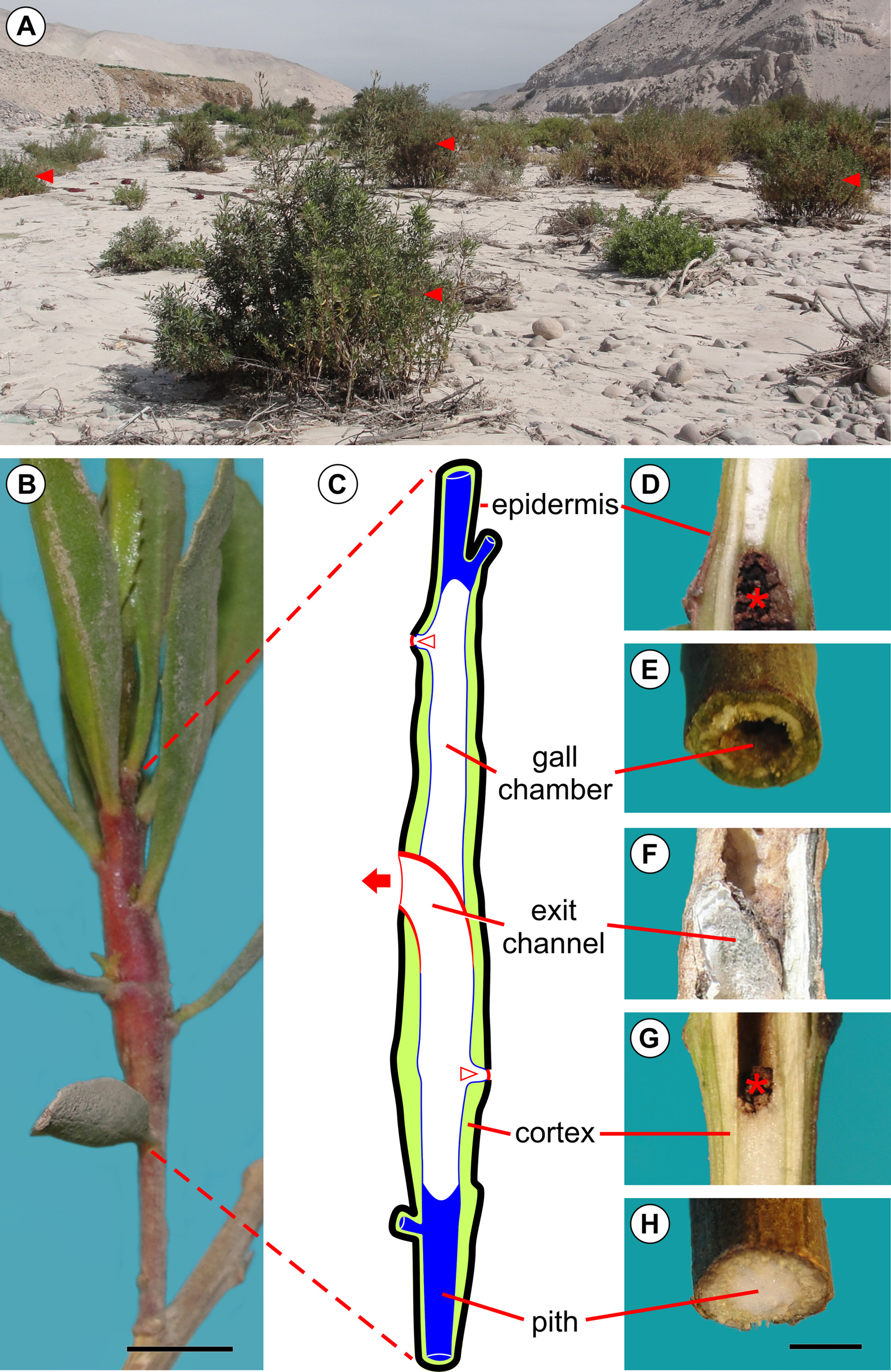
Host plant and distribution: Baccharis salicifolia ( Fig. 7 View FIGURE 7 A) is the only host plant known for E. azapaensis . The moth is known only from the type locality, the Azapa Valley, Atacama Desert, northern Chile. B. salicifolia is native to sage scrub and desert communities of the southwest United States and northern Mexico, and widespread in the Neotropics.
Life history: Young galls induced by E. azapaensis are typically found on young buds of the terminal branch of the host plant ( Fig. 8 View FIGURE 8 A). Eggs were not found during the fieldwork but are presumably laid on the young buds, which will be subsequently galled by the young larva. The larva feeds on the inner tissues (pith) of the branch, leaving the outer portions (cortex) almost intact ( Fig. 7 View FIGURE 7 C–H); a few feeding scars can be found on the internal surface of the cortex. Only one larva is typically found in each gall. A typical mature gall induced by E. azapaensis is fusiform and widest at the middle ( Fig. 7 View FIGURE 7 B–C). The larva constructs two orifices for discharging feces, one near the base of the gall and another near the apex ( Fig. 7 View FIGURE 7 C, 8B–D). The holes are covered with a silken layer that is sealed with a black oral secretion. Thus, the internal chamber of the gall is not filled with feces, with only a few remaining inside ( Fig. 7 View FIGURE 7 D, G). An occasional gall has more than two of these orifices. The larger opening in a mature gall is the operculum ( Fig. 7 View FIGURE 7 C, G, 8G–I), which is typically located near the middle of the gall. It is cut by the larva prior to pupation, resulting in a thin layer of bark that covers the end of the exit channel transversely. The operculum is identifiable from outside as a broad, omega-shaped area whose margin is internally continuous with the wall of the exit channel ( Fig. 7 View FIGURE 7 G). The pupa always has the head positioned upward, and the terminalia downward ( Fig. 8 View FIGURE 8 F). In the field, pupal exuvia are occasionally found partially extruded from the gall ( Fig. 8 View FIGURE 8 H), indicating that an adult has recently exited the gall. In older galls, the pupal exuvium and operculum are commonly lost ( Fig. 8 View FIGURE 8 I).
Molecular phylogeny: A total of 658 nucleotide sites were analyzed for Eugnosta and Aethes species, and 109 (16%) of these were variable. In accordance with the hypothesis proposed here, E. azapaensis was recovered as monophyletic, sister to other species within Eugnosta , in both methods of inference ( BI and ML) with full branch support ( Fig. 9 View FIGURE 9 ). The evolutionary divergence between E. azapaensis and the sister clade ( Eugnosta sp. + E. deceptana ) was 9% (± 2%), and the K2P distance between species of Eugnosta was 5% (± 2%) ( Fig. 9 View FIGURE 9 ), indicating a high degree of variation within this lineage.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.