Scydmaenus (Kingius) carinatus (King)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5371.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D60B50D1-280B-4403-9E5B-25C0704A43A1 |
|
DOI |
https://doi.org/10.5281/zenodo.10166437 |
|
persistent identifier |
https://treatment.plazi.org/id/3E380C57-FFE8-4A61-27AC-B4ECFF0CE267 |
|
treatment provided by |
Plazi |
|
scientific name |
Scydmaenus (Kingius) carinatus (King) |
| status |
|
Scydmaenus (Kingius) carinatus (King) View in CoL
Heterognathus carinatus King, 1864: 97 .
Scydmaenus (Cholerus) carinatus (King) View in CoL ; Csiki, 1919: 72.
Scydmaenus (Heterognathus) carinatus (King) View in CoL ; Franz, 1975: 275.
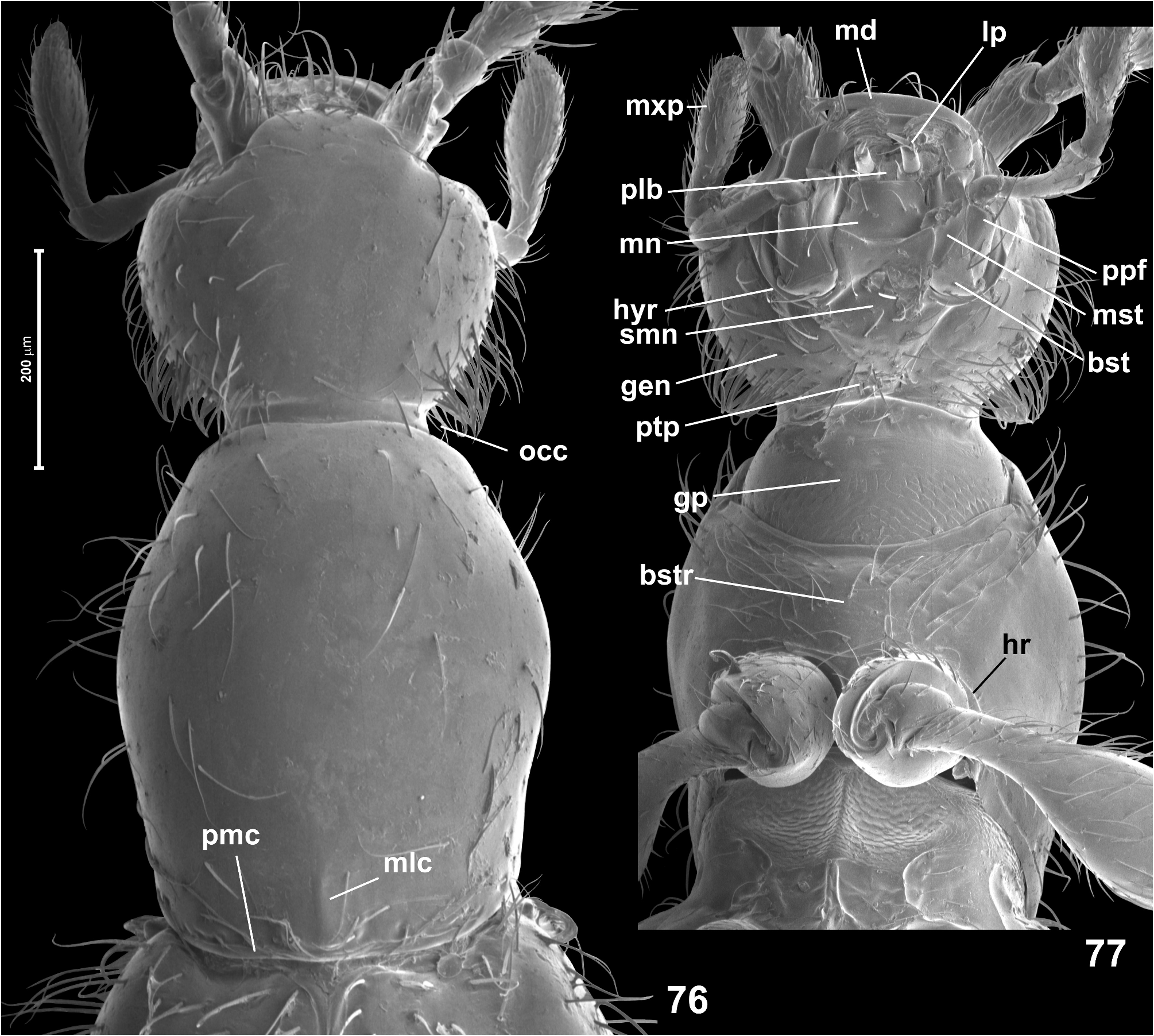
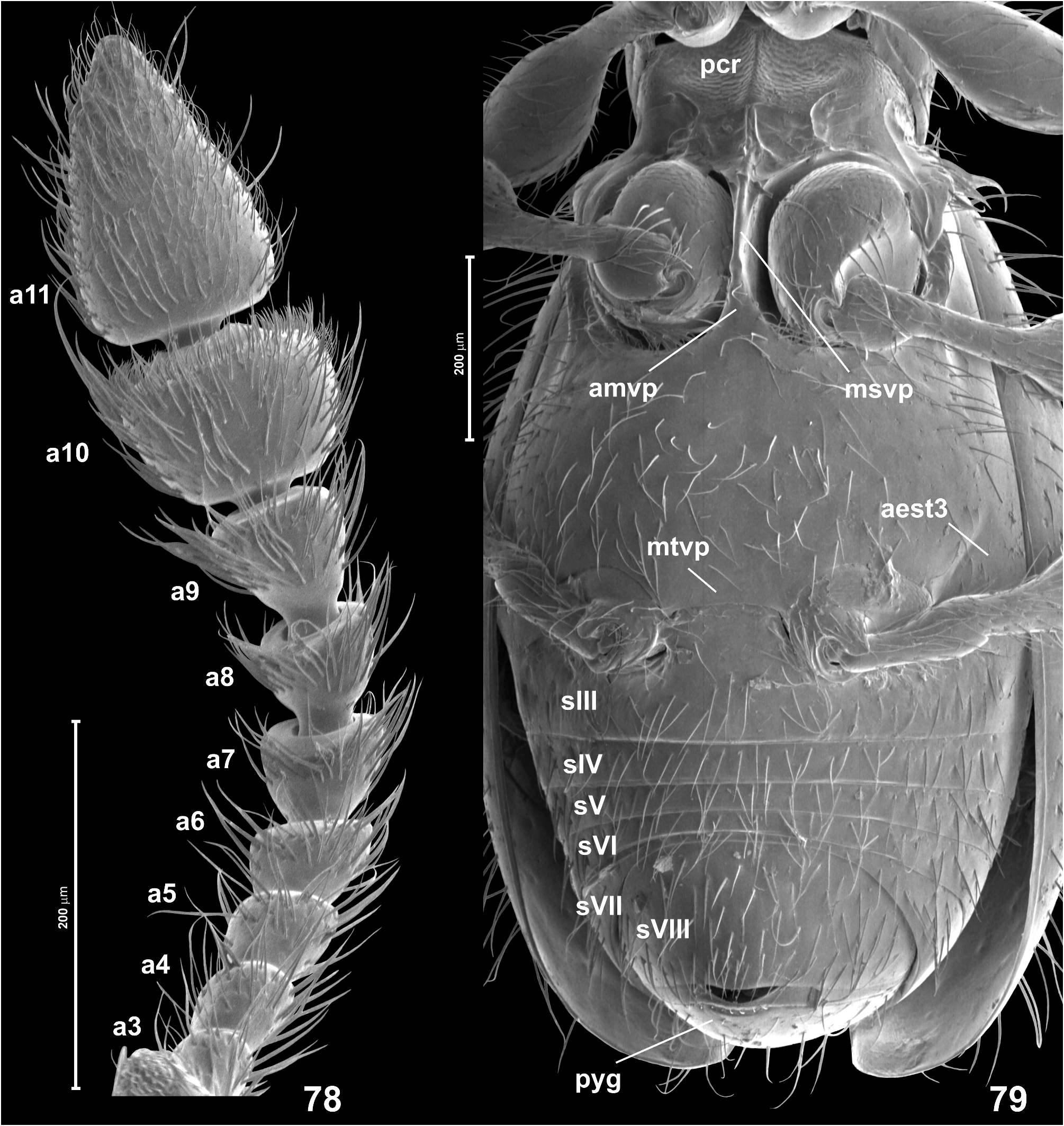
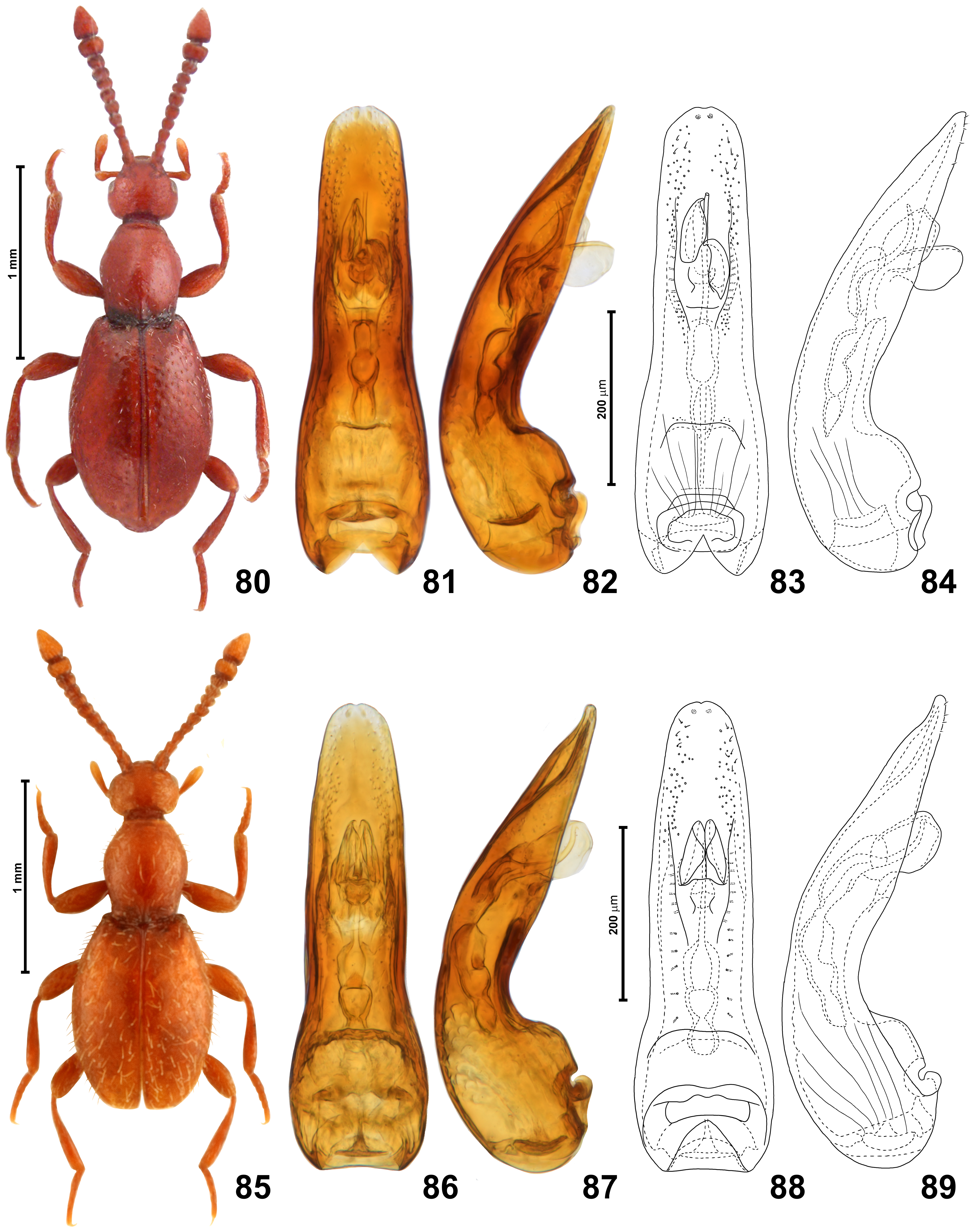
( Figs 76–89 View FIGURES 76–77 View FIGURES 78–79 View FIGURES 80–89 , 195 View FIGURES 189–205 )
Franz’s (1975) redescription. Franz redescribed this species based only on non-type specimens preserved at BNHM. They slightly differ in finer elytral punctures from the syntype female available for my study. Males showing similarly large elytral punctures as the female syntype, a male from BNHM studied by Franz with fine elytral punctures, and additional non-type specimens with fine elytral punctures were studied, and their aedeagi do not show any differences that could justify considering the different elytral punctation anything but an intraspecific variation. However, there is a chance that specimens with strong elytral punctures (referred below as ‘true S. carinatus ’) and those with fine punctures (referred below as ‘ S. carinatus sensu Franz’) are different species (see also Remarks below), and it is important to make a distinction to facilitate solving this problem. Listing all studied specimens without differentiating them can make it difficult to address this issue in future. For this reason, the studied material is divided into two subgroups, in order to keep track of specimens representing both variants.
Scydmaenus carinatus with strong elytral punctures (‘true S. carinatus ’). Type material studied. Lectotype (here designated) ( AUSTRALIA), ♀ ( Fig. 80 View FIGURES 80–89 ), with labels illustrated in Fig. 195 View FIGURES 189–205 : “K23214” [brownish, handwritten], “ Heterognathus / carinatus / 154” [brownish, handwritten], “K 197779” [white, printed], “SYNTYPE” [yellow, printed] ( AMS).
Additional material studied: NEW SOUTH WALES: 1 ♂, 1 ♀, Central Mangrove, Central Coast, 09.03.1991, in detritus under rotten log, in and around opening to ant nest in woodland, leg. V. Lorimer ( ANIC, cPJ); 2 exx. on one card lacking any data except presumably Lea’s label with “Paramatta” and misidentified as Heterognathus princeps , labelled as topotype by S. Misko in 1976, Sydney ( ANIC, on permanent loan from Macleay Museum, University of Sydney ) .
Scydmaenus carinatus with fine elytral punctures (‘ S. carinatus sensu Franz’). Material studied. SOUTH AUSTRALIA: 1 ex., Gawler, A.M. Lea, identified as S. carinatus by H. Franz ( SAMA) ; WESTERN AUSTRALIA: 4 exx., Swan River , Lea ( SAMA) ; VICTORIA: 1 ♂, 1 ♀, Sea Lake, Goudie , “1910—172”, identified as “ Heterognathus carinatus King vic.” by A. M. Lea and as Scydmaenus carinatus King by H. Franz ” ( BNHM) ; 2 exx., Ocean Grove , correctly identified by Lea ( SAMA) . Three more historical specimens from SAMA were studied, but with illegible labels or lacking labels.
Revised diagnosis. In both sexes, antennomere 10 distinctly transverse ( Fig. 78 View FIGURES 78–79 ); BL> 1.7 mm; aedeagus in dorsal view narrowing in distal half, with apical margin much narrower than basal margin.
Redescription. Body of both sexes ( Fig. 80 View FIGURES 80–89 ) strongly convex, elongate and slender, BL 1.80–1.88 mm; pigmentation uniformly light to moderately dark brown (including appendages), in dark specimens with somewhat reddish hue; cuticle moderately glossy, covered with vestiture of setae slightly lighter than body.
Head ( Figs 76–77 View FIGURES 76–77 ) in dorsal view transverse and somewhat subhexagonal, broadest at eyes, HL 0.30–0.33 mm, HW 0.35–0.36 mm; vertex and frons confluent and weakly convex, posterior margin of vertex nearly straight; tempora about twice as long as length of eye in dorsal view; supraantennal tubercles indistinct; frons over antennal fossae broadly subtriangular and with rounded anterior margin. Eyes small, oval, not emarginate posteriorly and oblique in relation to long axis of head. Punctures on frons and vertex fine, inconspicuous; setae short, sparse, suberect, those on tempora distinctly denser and more erect than those on frons and vertex. Genae ( Fig. 77 View FIGURES 76–77 ) as sparsely setose as frons and vertex, except for dense setae on lateral regions. Anterior (exposed) region of head capsule demarcated from neck region by short abrupt impression around occipital constriction, anterior margin of gular plate on neck region lacking anteriorly-directed projection. Submentum ( Fig. 77 View FIGURES 76–77 ) without submental lobes; hypostomal ridges ( Fig. 77 View FIGURES 76–77 ) extend mesally and anteriorly to connect at middle behind anterior margin of submentum, their median transverse portion distinct and strongly convex anteriorly. Antennae ( Figs 78 View FIGURES 78–79 , 80, 85 View FIGURES 80–89 ) moderately long and slender, AnL 0.88–0.90 mm; two terminal antennomeres forming moderately sharply delimited club; scape twice as long as broad, distinctly broadening distally; pedicel twice as long as broad; antennomere 3 indistinctly elongate, 4–5 each about as long as broad, 6–8 each indistinctly transverse and each strongly broadening distally, 9 about as long as broad, also strongly broadening distally, 10 distinctly (but not strongly) transverse, 11 about as long as 9 and 10 combined, about 1.7 × as long as broad, nearly symmetrical.
Pronotum in dorsal view ( Fig. 76 View FIGURES 76–77 ) distinctly elongate, broadest slightly behind anterior third, PL 0.50–0.58 mm, PW 0.40–0.43 mm; anterior margin nearly straight; anterior corners weakly marked, obtuse-angled and blunt, lateral margins rounded; posterior corners obtuse-angled and blunt; posterior margin weakly arcuate; base with narrow and indistinct posterior marginal carina and with short and diffuse longitudinal median carina accentuated by diffuse and shallow impressions at both sides. Pronotal disc covered with fine but sharply marked, sparse punctures; setae slightly longer than those on head, moderately dense, suberect. Ventrally ( Fig. 77 View FIGURES 76–77 ) prothorax with nearly asetose and impunctate hypomera and basisternal region much longer than procoxal rests, sparsely covered with moderately long recumbent to suberect setae, with barely discernible anterior ‘collar’ and distinct vestiges of notosternal sutures visible as notches on sides of anterior prothoracic margin; hypomeral ridges distinct and complete, demarcating narrow inner (adcoxal) region of each hypomeron, anteriorly running along procoxal rests and connecting at middle to form anteprocoxal carina demarcating basisternal region posteriorly.
Elytra ( Figs 76 View FIGURES 76–77 , 80, 85 View FIGURES 80–89 ) from slightly rhomboidal to nearly evenly oval, broadest near middle, EL 0.98–1.00 mm, EW 0.73–0.75 mm, EI 1.33–1.34. Humeral calli small but distinctly elevated; basal impression on each elytron short and transverse, shallow; basal elytral foveae lacking; apices separately rounded. Elytral punctures shallow but distinct and dense, in some specimens (‘ S. carinatus sensu Franz’) fine and inconspicuous; setae distinctly longer and more erect than those on pronotum, moderately dense. Hind wings not studied.
Mesoventrite ( Fig. 79 View FIGURES 78–79 ) with carinate subrectangular mesoventral intermesocoxal process posteriorly fused with short and posteriorly broadening anterior metaventral process, fusion site weakly marked on surface. Metanepisterna ( Fig. 79 View FIGURES 78–79 ) fused with metaventrite, only vestiges of demarcating sutures visible near anterior and posterior margins of metathorax. Metaventral intermetacoxal process ( Fig. 79 View FIGURES 78–79 ) broad and short, with weakly concave posterior margin, its lateral portions form short subtriangular processes weakly projecting posteriorly; distance between metacoxae subequal to 1/3 width of metaventrite at its posterior margin and as wide as one metacoxa. Metaventrite ( Fig. 79 View FIGURES 78–79 ) weakly convex, slightly flattened at middle, unmodified and evenly covered with sparse, short, slightly suberect setae.
Legs ( Figs 77 View FIGURES 76–77 , 79 View FIGURES 78–79 , 80, 85 View FIGURES 80–89 ) moderately long and slender; unmodified, except for slightly broadened proximal region of protarsi bearing tenent setae on tarsomeres 1–3. Protarsomere 1 about twice as long as broad, 2–4 each indistinctly elongate, 5 about 3 × as long as broad; meso- and metatarsi of subequal length, both distinctly longer that protarsi, each with tarsomere 1 nearly 3 × as long as broad, tarsomeres 2–4 each weakly elongate, and tarsomere 5 about 3 × as long as broad.
Aedeagus ( Figs 81–84, 86–89 View FIGURES 80–89 ) elongate and slender, AeL 0.53 mm, in dorsal view median lobe broadest near proximal 1/4, narrowing distally, apex broadly rounded and with indistinct and narrow median emargination; flagellum broadened in proximal region to form three consecutive symmetrical chambers; median lobe with fine setae in apical dorsal region; ostium situated in distal half of median lobe, far from its apex.
Female. Externally similar to male, except for slenderer protarsi lacking ventral tenent setae. BL 1.80–1.88 mm; HL 0.30–0.33 mm, HW 0.35–0.36 mm, AnL 0.88–0.90 mm; PL 0.50–0.58 mm, PW 0.40–0.43 mm; EL 0.98–1.00 mm, EW 0.73–0.75 mm, EI 1.33–1.34.
Distribution. S Australia: CE New South Wales, NW and CS Victoria, SE South Australia, and SW Western Australia.
Remarks. The two ‘forms’ that differ in the elytral punctures, mentioned in the first paragraph of this species’ redescription (the ‘true S. carinatus ’ with strong punctures, and ‘ S. carinatus sensu Franz’ with fine punctures), do not differ in proportions of antennomeres and other body parts, and have almost identical aedeagi ( Figs 81–84 View FIGURES 80–89 vs. 86–89). The type specimen(s) come from Paramatta in New South Wales, and the additional specimens of the ‘true S. carinatus ’ are also from the vicinity of Sydney. Studied specimens of the ‘ S. carinatus sensu Franz’ have been collected in Victoria (near Melbourne and in Sea Lake), South Australia (near Adelaide), and Western Australia (near Perth). The number of specimens is too small to draw any general conclusions concerning the distribution of these forms. At least some specimens of both forms (including the type material) were collected in nests of ants (according to King, 1864: “This species was discovered under the bark of a dead “Stringy Bark”, in the nest of small black ants”). The very similar S. formicarum that belongs in the same subgenus was also collected from ant colonies. The identity of these ants remains unknown. Morphologically similar species of myrmecophiles may be associated with only one ant host, and it is possible that the forms that differ in elytral punctures have strict preferences towards a particular ant species. It is important to collect more specimens together with their ant hosts, and to correlate morphological features with the distribution and host preferences. A molecular analysis could also help solving this interesting problem. Tentatively, both forms are treated as representing one variable species.
Scydmaenus carinatus is similar to S. formicarum . It is easy to distinguish these species by differences in the body length ( S. carinatus is clearly larger), proportions of antennomeres ( S. carinatus has the antennomere 10 distinctly transverse vs. as long as broad in S. formicarum ), and the shape of the median lobe of the aedeagus in dorsal view.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scydmaenus (Kingius) carinatus (King)
| Jałoszyński, Paweł 2023 |
Scydmaenus (Heterognathus) carinatus (King)
| Franz, H. 1975: 275 |
Scydmaenus (Cholerus) carinatus (King)
| Csiki, E. 1919: 72 |
Heterognathus carinatus
| King, R. L. 1864: 97 |