Sarotherodon knauerae, Neumann, Dirk, Stiassny, Melanie L. J. & Schliewen, Ulrich K., 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.203566 |
|
DOI |
https://doi.org/10.5281/zenodo.5671855 |
|
persistent identifier |
https://treatment.plazi.org/id/3F289856-720C-FF9C-4ABD-FB7B640CF954 |
|
treatment provided by |
Plazi |
|
scientific name |
Sarotherodon knauerae |
| status |
sp. nov. |
Sarotherodon knauerae View in CoL , sp. nov.
Figures 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 8 View FIGURE 8 ; Table 3
Holotype. ZSM 29924, male 75.2 mm SL; Cameroon: South-West Prov.: Lake Ejagham, (Cross River basin), Manyu Subdivision; 5° 45' N / 8° 59' E; February-March 1993, U. K. Schliewen. Paratypes. 60 specimens, 76.7– 29.0 mm SL; same data as holotype. AMNH 233728 (2, ex ZSM 29928), 52.8–60.9 mm SL. ANSP 188864 (2, ex ZSM 29928), 52.2–56.7 mm SL. MRAC 2004-04-P-1-2 (2, ex ZSM 29928), 59.2–59.5 mm SL. ZSM 29925 (11), 52.5–76.7 mm SL. ZSM 29926 (10), 54.9–73.7 mm SL. ZSM 29927 (5), 29.0– 39.6 mm SL. ZSM 29928 (34 now 28), 45.0– 63.6 mm SL. Non-type material. AMNH 251428 (2 C&S, ex ZSM 27683), 38.2–48.2 mm SL. ZSM 30464 (12), juveniles 41.6–50.9 mm SL; same data as holotype.
Diagnosis. Sarotherodon knauerae sp. nov. is distinguished from all congeners by the possession of an inflated second pharyngobranchial element in the upper pharyngeal jaw, and is one of the smallest known Sarotherodon , reaching a maximum observed size of only 75.2 mm SL. It further differs from sympatric S. lamprechti sp. nov. in possessing a terminal (vs. prognathous) mouth, scaled pectoral fin base, short pectoral fins ending at or in front of anus (vs. reaching first anal fin-spine or beyond) and short pelvic fins ending well in front of anus (vs. reaching anus). Additionally, S. knauerae possesses a shorter upper lip (19.1–25.4 vs. 22.5–28.4 % HL), a higher total gill raker count (24–30, mode 28 vs. 20–25, mode 22), and larger eyes (24.0–31.2 % HL, mode 27.3 vs. 20.4–29.2 % HL, mode 22.8). Distinguished from neighbouring riverine S. galilaeus populations (Cross, Wouri & Lower Niger), S. g. multifasciatus and S. g. borkuanus by a shorter anal fin (12.0–14.6 % SL vs. 15.3–19.3 % SL / 15.1–25.7 % SL and 13.4–17.9 % SL) and narrower caudal peduncle (12.8–14.5 % SL vs. 16.0–18.7 % SL / 13.9–18.3 % SL and 13.6–17.4 % SL). It differs from S. caroli and S. linnellii in higher gill raker counts (24–30 mode 28 vs. 18–20 mode 19 / 15–18 mode 18), from S. g. sanagaensis in a lower preorbital depth (17.8–23.0 vs. 25.0–27.8 % HL) and from S. g. boulengeri and riverine Sarotherodon galilaeus in a lower body depth (36.5–43.9 vs. 44.5–50.0 % SL and 43.5–44.6 % SL). Finally it differs from all other Lake Barombi Mbo Sarotherodon in a higher number of dorsal fin-rays (13 or 14, mode 13 vs. 10–12, mode 11).
Description. Based on holotype and 60 paratypes. For morphometric and meristic data refer to Table 3, for general appearance see Figs. 4 View FIGURE 4 A&B and 6A&B. Sarotherodon knauerae is one of the smallest known Sarotherodon (maximum size 75.2 mm SL, holotype, mature male). Snout acute, mouth terminal, lips thickened but not fleshy. Head profile straight, sharply convex behind nape to dorsal fin; ventral head profile convex. Greatest body depth between insertion of dorsal and pelvic fins. Caudal peduncle deeper than long.
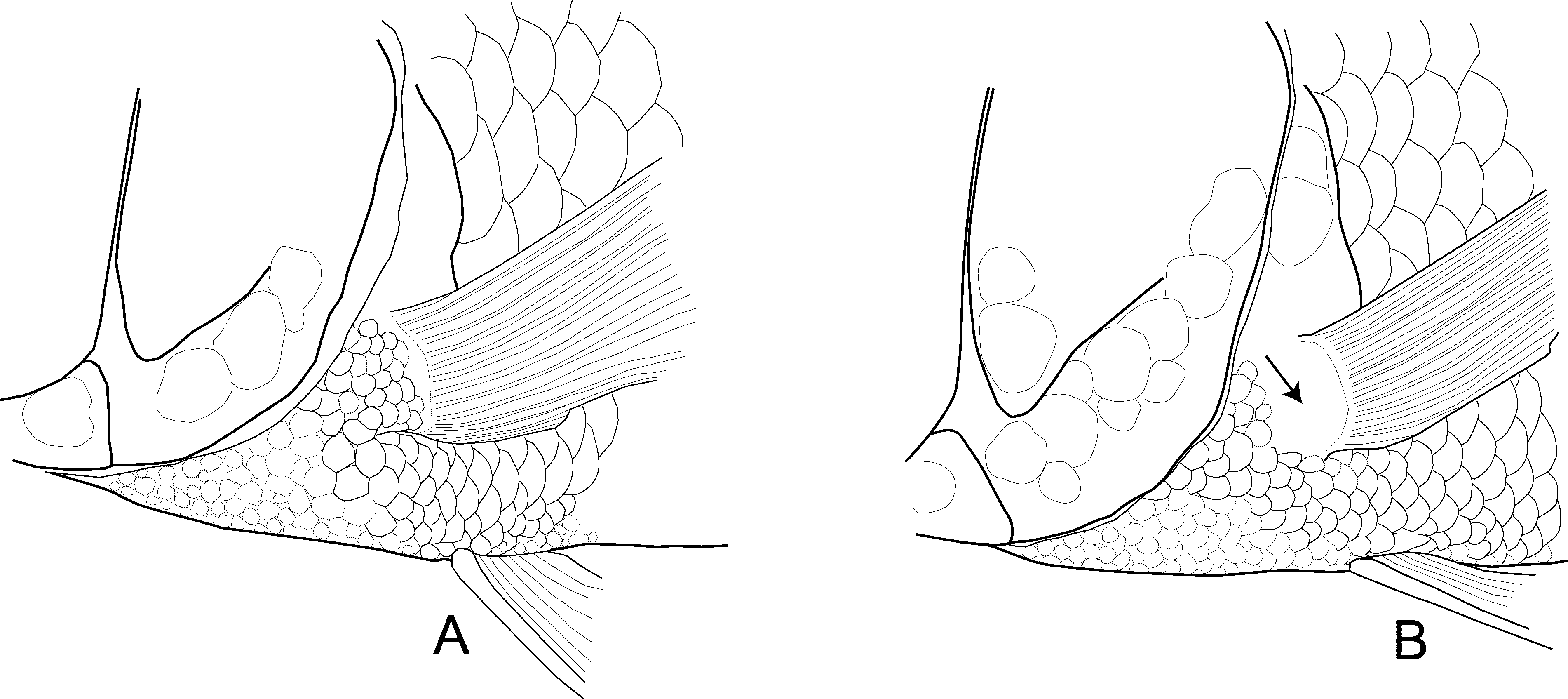
Squamation. Two scale rows on cheek, two additional scales dorso-rostrally; opercle irregularly covered with scales increasing in size towards dorso-caudal corner. Small cycloid scales cover chest, pectoral-fin base ( Fig. 5 View FIGURE 5 A), and interpelvic area; relatively abrupt size transition behind pelvic-fin base to ventral flank scales, which are nearly twice as large as chest scales. Belly covered with small circular scales; minute scales around anus and at spiny anal fin-base. Upper branch of lateral line ascending to spiny dorsal fin and separated from it by 3 scale rows, ending 1– 1.5 scale rows below fin and separated from lower lateral line by two scale rows; lower lateral line beginning above level of second anal fin-spine and extending onto caudal fin (mean of two pored scales on caudal fin). Caudal finrays except distal fifth finely scaled along fin rays, squamation of basal parts not restricted to fin rays.
Fins. Dorsal fin XV or XVI (mode XVI) spines, 13 or 14 (mode 13) branched rays. Anal fin III spines, 10–12 (mode 11) branched rays. Dorsal fin increasing in height to 5th spine, following spines of equal length. Dorsal fin membranes ending below tip of spines (females) or at tip and slightly produced (males). Rayed dorsal and anal fins rounded, reaching caudal-fin base. Pelvic fin-spine about half the length of first branched ray, fin pointed but not produced, ending well in front of anus. Pectoral fin pointed, branched rays increasing in length to fourth ray, and then gradually decreasing in length. Caudal fin truncated and deeper than long.
Gill rakers. Total rakers on first arch 24–30 (mode 28). Rakers stout, truncated distally (not slender and elongate as in most S. galilaeus populations); first ceratobranchial rakers small, increasing in length from fifth to ninth raker, then equal in length. Gill raker in angle of arch and first four epibranchial rakers slender, strongly decreasing in size towards last.
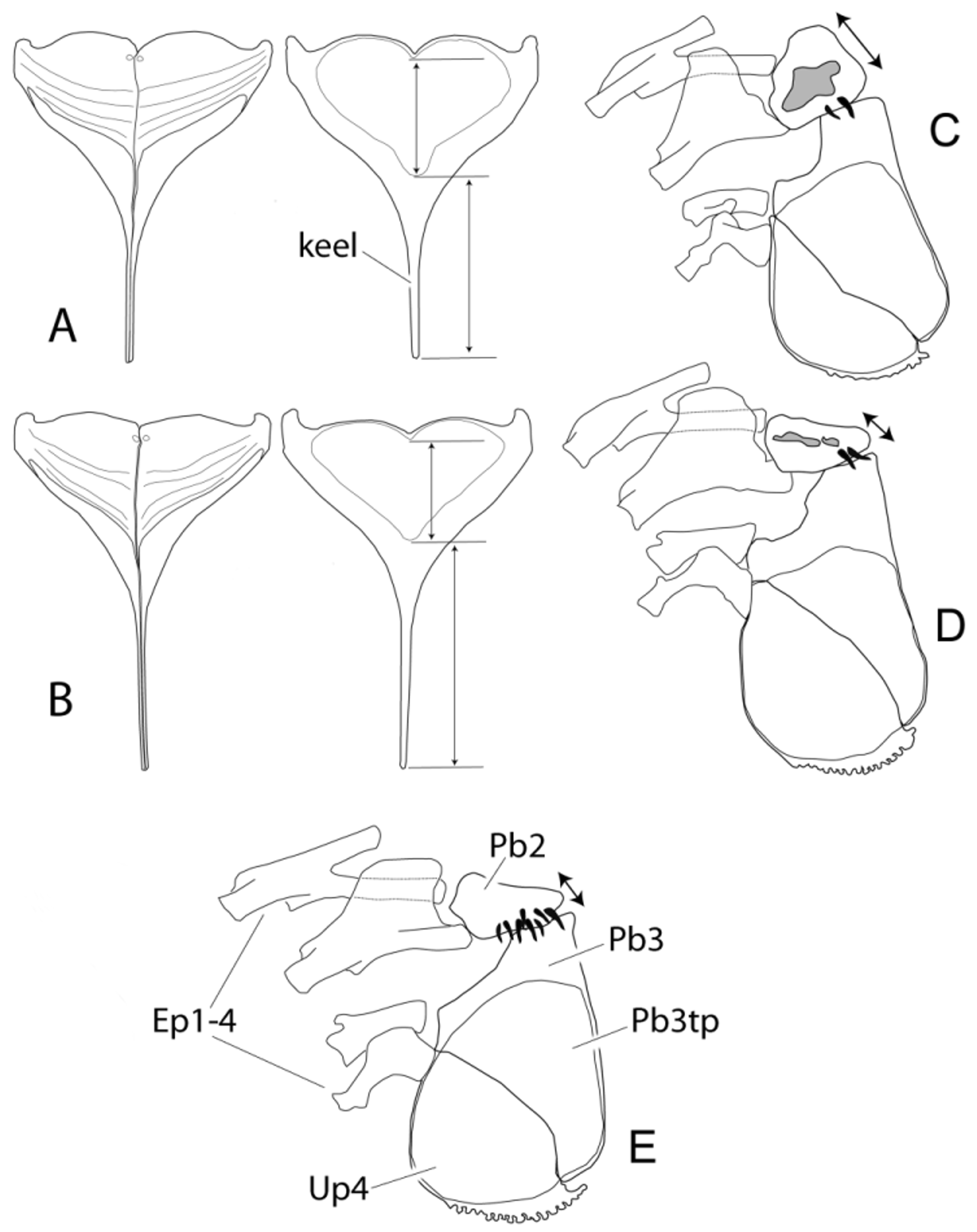
Jaws and dentition. Jaws isognathous with 44–81 (mean 63) slender necked bicuspid outer-row teeth on upper and 32–50 (mean 41) on lower jaw. Outer row teeth with laterally compressed cusps and deeply embedded in the soft tissue (up to the crown); single posterior outer row teeth tricuspid. Two rows of tricuspid inner teeth on both jaws. Lower pharyngeal jaw relatively stout, ventral keel relatively short, less than twice the length of dentigerous area ( Fig. 8 View FIGURE 8 A). Dentigerous area covered with fine unicuspid teeth, crowded and brush-like in the posterior part of the tooth plate. Second pharyngobranchial element of upper pharyngeal jaw inflated and vacuolated, bearing two or three robust unicuspid teeth on posterior face ( Fig. 8 View FIGURE 8 C).
Colour in life ( Fig. 6 View FIGURE 6 A–D). Males: Base body colour golden, sometimes with metallic golden-green hue. Metallic hue most evident on upper lip and cheek. Lateral bands present in juveniles and subadults, in adult males sometimes visible as irregular blotches or dots. Additional irregular black blotches may be present along dorsal-fin base. Nuptial males have black lower jaws, lower parts of opercular region, chest and belly extending back to anal fin and along anal fin insertion, including pelvic fins and pectoral fin insertion. Iris dark greyish black with golden inner rim. Dorsal fin golden-yellowish, free tips of dorsal spines and distal margin of rayed fin black. Caudal fin golden-grey medially, dusky greyish towards the lobes, ventral lobe almost black in nuptial males.
Females: Overall body colour light grey with metallic bluish-golden iridescence laterally on flanks and head; golden iridescence on cheek and opercle. Iris clear or slightly yellowish with golden inner rim. Upper lip metallic blue, lower lip and ventral head and belly white to light grey. Dorsal fin transparent with distinct golden iridescence and narrow dark outer margin. Caudal fin yellowish-golden medially becoming transparent distally. Anal fin yellowish-white, pelvic fins reddish near fin insertion and white distally. Colour in preservation ( Fig. 4 View FIGURE 4 A&B). Males: Overall brownish. Lips dark brownish grey, ventral part of lower lip blackish. Cheek pale brown, opercle with faint opercular blotch at distal corner. Branchiostegal membranes brownish to dark brown. Six dark brown bands starting one scale below upper lateral line and extending onto belly. Belly and chest laterally dark brown, white ventrally between pelvics and anus. Dorsal fin membrane distally beige, produced lappets and sometimes additional parts of the fin dark brown to blackish; rayed portion of fin beige brown, with five to six faint irregular brown stripes. “ Tilapia spot” present as faint brown blotch over base of last dorsal spine. Caudal fin beige with brownish membranes between rays. Anal fin beige, becoming brownish near fin base, some males with a broad dark brown to black band near fin base. Pelvic fins dusky brown and strongly pigmented. Pectoral fins beige brownish and translucent, pectoral fin base strongly pigmented.
Females: Overall colouration pale brown. Lips light beige to greyish, lip fold of upper jaw brownish grey, ventrally lower lip greyish. Interorbital pale to dark brown, nape and region in front of dorsal fin insertion brown. Cheek brownish, upper part of the opercle dark brown, fusing with opercular blotch at distal corner, ventrally opercle and branchiostegal membranes beige. Vertical bars faintly visible on flanks, their vertical expansion restricted to area of lower lateral line. Chest brownish, belly laterally pale greyish, black abdominal wall, white ventrally between pelvics and anus. Dorsal fin membrane beige distally, lappets and sometimes additional parts of fin brownish, with brownish markings near fin base, rayed fin beige brown, with four faint irregular brown stripes. “ Tilapia spot” not visible. Caudal fin beige with brownish membranes between rays. Anal fin beige with conspicuous brown band near fin base in some females. Pelvic fins beige, pectoral fins pale brown and translucent, pectoral fin base pigmented.
Distribution and ecology. Sarotherodon knauerae is a gregarious, predominantly benthic species, endemic to Lake Ejagham in the Cross River drainage ( Cameroon). It is present in all habitats and at all depths, but appears to be most common inshore (to 3 m) than in deeper zones. Presence of numerous juveniles in the dry season (January/ February) suggests that reproduction takes place predominantly in the rainy season (August/September). Adults in nuptial colouration are commonly observed in the rainy season and less frequently in the dry season. The species is a detritivore, taking detritus from both soft and hard substrates (see Fig. 6 View FIGURE 6 C) as well as from the water surface. Males do not vigorously defend courtship territories with a bower. In aquaria S. knauerae are maternal mouth brooders, however, spawning and brood care has not been observed in the field.
Etymology. Named for Mrs. Barbara Knauer, former technician at the Max-Planck-Institut (Seewiesen), who substantially supported UKS as a technician and friend during his PhD studies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.