Ilyobius hauseri (Contrera-Ramos, Fiorentin & Urakami, 2005)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5165.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:99B81CA6-28B2-4678-84BF-53B6F891B388 |
|
DOI |
https://doi.org/10.5281/zenodo.6948562 |
|
persistent identifier |
https://treatment.plazi.org/id/3F611B55-FFA0-176A-E1BE-FCE2FAACF990 |
|
treatment provided by |
Plazi |
|
scientific name |
Ilyobius hauseri (Contrera-Ramos, Fiorentin & Urakami, 2005) |
| status |
|
Ilyobius hauseri (Contrera-Ramos, Fiorentin & Urakami, 2005) View in CoL
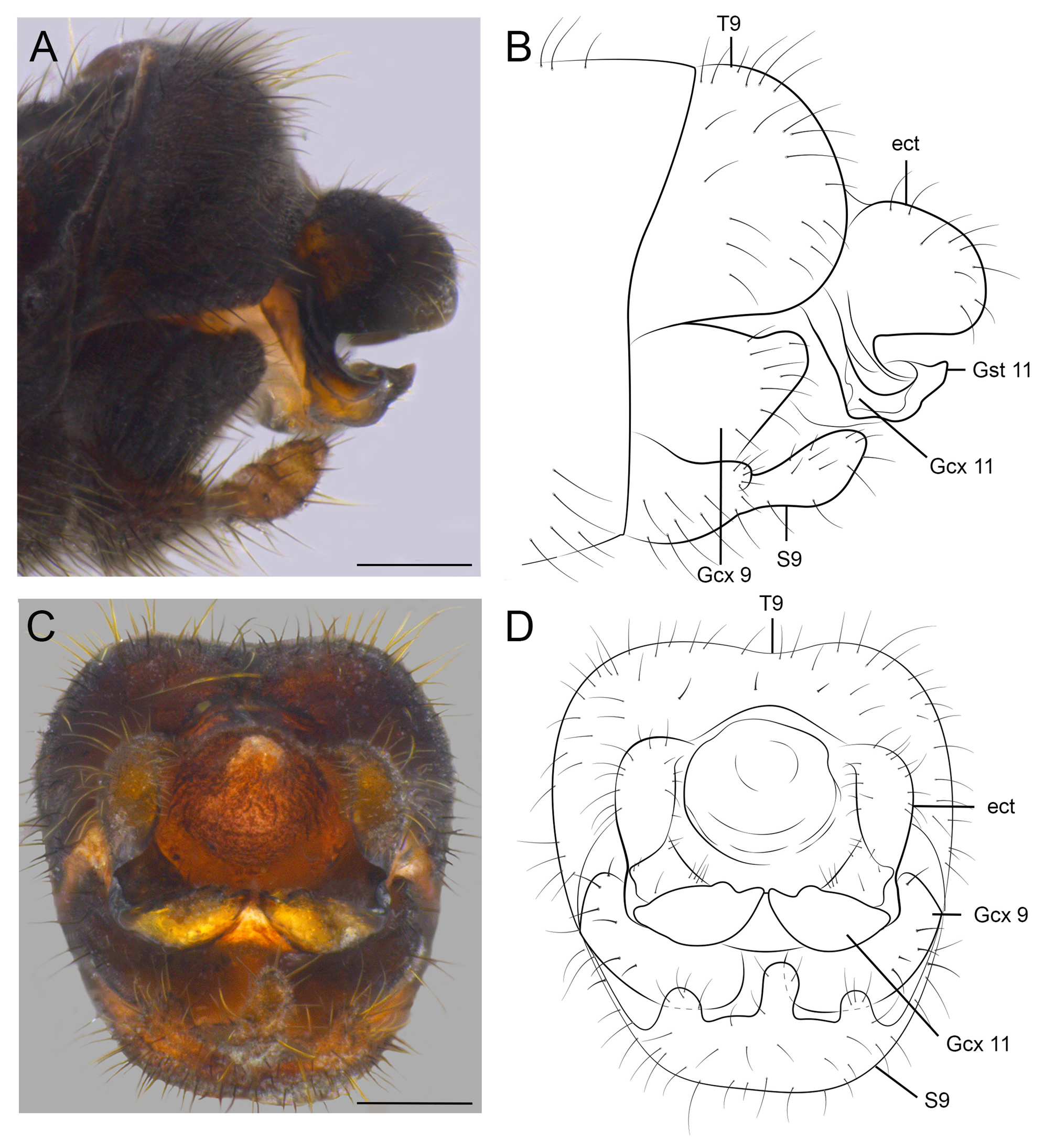
( Figs 4C–E View FIGURE 4 ; 11–12 View FIGURE 11 View FIGURE 12 )
Protosialis hauseri Contreras-Ramos et al. 2005: 268 View in CoL (original description); Contreras-Ramos 2008: 808 (taxonomy).
Ilyobius hauseri View in CoL ; Liu et al. 2015a: 31 (phylogeny of Sialidae View in CoL ; comb. nov.); Liu et al. 2015b: 55 (key of species); Oswald 2018 (online catalog); Ardila-Camacho et al. 2021: 48 (taxonomy); Rafael et al. 2022 (online catalog).
Material examined. Holotype: male genitalia, Brazil; Rio Grande do Sul, Floresta Nacional de São Francisco de Paula , (29°02’S; 50°23’W, 930 m) 17.VIII.2003 (larva collected) - 20.IX.2003 (adult emerged in laboratory), Y. Urakami & G.L. Fiorentin cols. ( MCNZ) GoogleMaps . Paratype: female genitalia, same data as holotype. Additional specimens: same as holotype, except the following data: seven males, three fixed in 80% alcohol, four pinned; three females, two fixed in 80% alcohol, one pinned, 30.VIII.2015 (larva collected)— 02.IX.2015 (adult emerged in laboratory), N. Hamada, C. Benetti, G. Dantas, A.M.O. Pes cols. ( INPA) .
Male genitalia redescription ( Figs 6C–E View FIGURE 6 ; 11A–D View FIGURE 11 ). Tergite 9 sclerotized, sparsely setose; in lateral view, subtriangular with rounded margin ( Fig. 11A, B View FIGURE 11 ); in dorsal view, sub-rectangular; basal margin concave, distal margin convex. Sternite 9 trifurcate; sparsely setose; central projection slightly longer than lateral ones ( Fig. 11B View FIGURE 11 ); in lateral view, central projection with similar width along entire length ( Fig. 11B View FIGURE 11 ). Endophalic sac membranous, eversible, with several fringed thorny setae ( Fig. 6C–E View FIGURE 6 ). Gonocoxite 9 robust, setose; in lateral view, subtriangular, with rounded apex, dorsal margin slightly convex ( Fig. 11A, B View FIGURE 11 ). Anal tubercle membranous. Ectoprocts paired, in lateral view, rounded; dorsal margin convex; ventral margin straight, directed downwards with proximal margin fused to gonocoxite 11 ( Fig. 11B View FIGURE 11 ); in caudal view, subcylindrical ( Fig. 11D View FIGURE 11 ). Gonocoxite 11, in lateral view, L-shaped, distal region rounded at apex and projected downwards representing gonostylus 11 ( Fig. 11B View FIGURE 11 ); gonocoxite 11 divided into two sclerites in caudal view, medially directed and connected by a membranous region ( Fig. 11D View FIGURE 11 ); each sclerite with internal margin straight. Gonostylus 11, in lateral view, distally rounded and projected downwards.
Female genitalia redescription ( Fig. 12A–E View FIGURE 12 ). Sternite 7 with posteriorly projected, thumb-shaped, posteromedian projection in lateral view ( Fig. 12A, B View FIGURE 12 ); in ventral view, subpentagonal, with a tubercular projection medially ( Fig. 12C, D View FIGURE 12 ). Tergite 9 with ventral region broadly valvate in lateral view; joined to upper region by a junction line ( Fig. 12A, B View FIGURE 12 ). Gonocoxite 8 reduced to small, unpaired, setose sclerite ( Fig. 12E View FIGURE 12 ), located beneath sternite 7 ( Fig. 12C, D View FIGURE 12 ). The gonapophyses 8 is present as a single, strongly sclerotized plate; in lateral view, subquadrate, dorsal margin convex proximally, concave distally ( Fig. 12A, B View FIGURE 12 ); in ventral view, subrectangular in shape, with anterior margin concave medially, posterior margin convex medially, lateral regions enlarged, with a sclerotized fold at the tip of each side ( Fig. 12C, D View FIGURE 12 ). Gonocoxite 9 subrectangular in lateral view, setose, posteriorly with small gonostylus 9 at apex ( Fig. 12A, B View FIGURE 12 ). Ectoproct short and ovoid in lateral view, setose ( Fig. 12A, B View FIGURE 12 ).
Remarks. No adults were collected in Malaise and Pennsylvania light traps installed at the stream’s banks where the new species larvae were collected. Ilyobius erebus sp. nov. is closely related to I. hauseri based on the morphology of the male and female genitalia, and to I. nubilus based on the morphology of the female genitalia. Male genitalia of I. hauseri and I. erebus sp. nov. share the subtriangular tergite 9 with rounded margins, the endophalic sac with several fringed thorny setae, and the trifurcated sternite 9, in which the median protrusion is elongate. In addition, the male gonocoxite 9 is subtriangular with rounded margins, and the ectoprocts have basal margin fused to gonocoxite 11 in both species. The L-shaped gonocoxite 11 constitutes a common characteristic of these two species. The female genitalia of I. erebus sp. nov., I. hauseri and I. nubilus have gonocoxite 8 reduced, representing a tiny, setose sclerite, located beneath sternite 7 ( Figs 7E View FIGURE 7 , 11E View FIGURE 11 ), while the two gonapophyse 8s form a single sclerotized plate ( Contreras-Ramos et al. 2005, Contreras-Ramos 2008, Liu et al. 2015a). However, the new species can be easily differentiated from I. hauseri and I. nubilus based on the following characters: in Ilyobius erebus sp. nov. the head is almost completely blackish, while in I. hauseri it is orange, with median, longitudinal, black band. The head in I. nubilus is black, slightly paler around the epicranial suture ( Contreras-Ramos 2008). Although these three species share the dark pronotum, I. hauseri has orangish-brown areas, which are absent in I. erebus sp. nov. and I. nubilus . In I. erebus sp. nov., the sternite 9 has the median projection wider medially ( Fig. 4A, B View FIGURE 4 ), while it has the same width along its entire length in I. hauseri ( Fig. 11B View FIGURE 11 ). Gonostylus 11 projects upwards distally in I. erebus sp. nov. ( Fig. 4A, B View FIGURE 4 ), whereas in I. hauseri it is distally projecting downwards ( Fig. 11B View FIGURE 11 ). The ectoprocts are subrectangular in dorsal view in I. hauseri , and the ventral margin is directed downwards in lateral view. By contrast, the ectoprocts are subtriangular, and the ventral margin is posteriorly directed in ventral view in I. erebus sp. nov. The female genitalia of I. hauseri , have gonapophysis 8 dorsally curved, with posterior margin strongly convex ( Contreras-Ramos et al. 2005), however, this structure is ventrally depressed and widened on the posterior half, with a broadly arched posterior incision in I. nubilus ( Contreras-Ramos 2008) . In I. erebus sp. nov., the two gonapophysis 8 appear as a single and straight sclerotized plate with anterior and posterior margins slightly convex, and the anterolateral corners are falcate ( Fig. 7C, D View FIGURE 7 ).
The larvae of Ilyobius erebus sp. nov. can be easily differentiated from I. chilensis (McLachlan, 1870) by the distinct general coloration of head and pronotum: the larva of I. chilensis has a distinct pattern of dark brown marks on the head, which are absent in the new species ( Fig. 8C View FIGURE 8 ) ( Archangelsky et al. 2017). Liu et al. (2015a) divided Ilyobius into two species groups (the I. chilensis group, and the I. mexicanus group). The first group is composed of I. chilensis , I. hauseri and I. nubilus , while the remaining species of Ilyobius , i.e., I. bimaculatus (Banks, 1920) , I. curvatus (Liu, Hayashi & Yang, 2015) , I. flammatus , I. flavicollis , I. mexicanus (Banks, 1901) , I. nigrocephalus Ardila-Camacho, Martins & Contreras-Ramos, 2021 , and I. ranchograndis (Contreras-Ramos, 2006) belong to the second group ( Liu et al. 2015a; Ardila-Camacho et al. 2021). Ilyobius erebus sp. nov. is placed in the I. chilensis group based on the morphology of the male sternite 9, which has an elongate median protrusion, the transversely band-like male gonocoxite 11 with short median processes, and the reduced female gonocoxite 8 ( Liu et al. 2015a).
Conservation. Land-use change is one of the main threats driving the decline in freshwater environments ( Sala et al. 2000). The natural vegetation in the region of the type locality of I. erebus sp. nov. has been replaced by Eucalyptus plantations; this fact directly and indirectly affects the aquatic biota through soil disturbance, which changes the limnological conditions ( Pozo et al. 1997). Eucalyptus leaves contain tannins and phenols that can change the chemical composition of water, reduce dissolved nutrients, and consequently prevent the colonization of organic matter by the decomposing microbiota ( Pozo et al. 1997).
The integrity and heterogeneity of the environment is a fundamental factor for colonization and permanence of aquatic insects ( Santos et al. 2020). Frequent and intense environmental impacts on these ecosystems can eliminate communities, especially in the case of species that have biological traits that disfavor more-active dispersive processes ( Poff et al. 2006). Ilyobius is a rare taxon with few occurrence points and weak dispersion ( Penny 1981, Contreras-Ramos 2008); therefore, the new species may be subject to habitat loss in the Morro do Ferro region.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ilyobius hauseri (Contrera-Ramos, Fiorentin & Urakami, 2005)
| Mendes, Gabriela Caroline, Nascimento, Jeane Marcelle Cavalcante Do, Fusari, Lívia Maria, Santos, Mireile Reis Dos & Hamada, Neusa 2022 |
Ilyobius hauseri
| Ardila-Camacho, A. & Rivera-Gasperin, S. L. & Martins, C. C. & Contreras-Ramos, A. 2021: 48 |
| Liu, X. & Hayashi, F. & Yang, D. 2015: 31 |
| Liu, X. & Hayashi, F. & Yang, D. 2015: 55 |
Protosialis hauseri
| Contreras-Ramos, A. 2008: 808 |
| Contreras-Ramos, A. & Fiorentin, G. L. & Urakami, Y. 2005: 268 |